The “ideal protein” concept is not ideal in animal nutrition
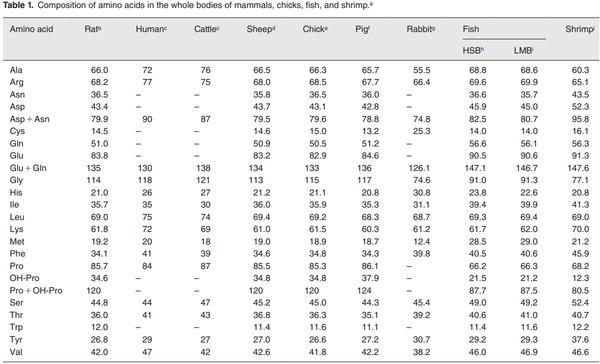
a Unless indicated otherwise, calculations were based on the molecular weights of intact AAs.
b Adult rats (60day-old in the postabsorptive state) without intestinal lumen contents.11
c Data for human fetuses (days 160–280 of gestation) and cattle (12-week old) were obtained from Davis et al.12 It was not reported whether calculations were based on the molecular weights of intact AAs or AA residues.
d Adult sheep (12-month old) without intestinal lumen contents.11
e Chickens (10-day old chickens in the postabsorptive state) without intestinal lumen contents.11
f Pigs (30-day old in the postabsorptive state) without intestinal lumen contents.11
g Fifty-three-day-old New Zealand White rabbits (males and females).5
h Juvenile hybrid-striped bass (HSB; 50g of body weight) without intestinal lumen contents.3
i Juvenile largemouth bass (LMB; 50g of body weight) without intestinal lumen contents.3
j Whole body of the whiteleg shrimp (Litopenaeus vannamei; 15g of body weight) without intestinal lumen contents.13
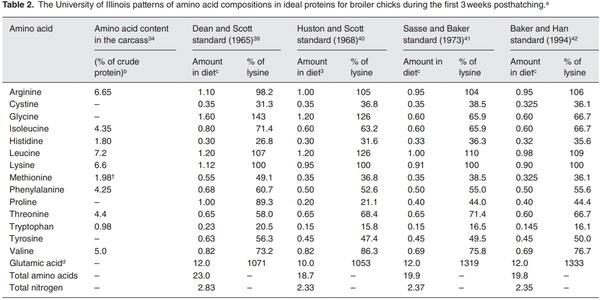
b Average values for 1-week-old and 4–5-week-old chicks.
c % of diet (as-fed basis; 90% dry matter).
d Provided as the nitrogenous source for synthesis of all NEAA in chicks.
†This value refers to l-methionine.
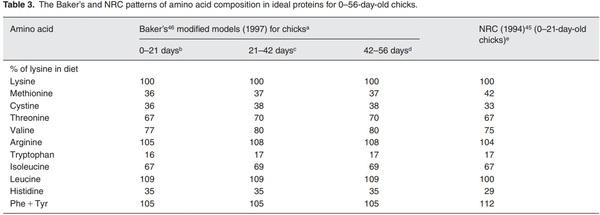
b Patterns of amino acid composition in the ideal protein are the same for male and female chickens. The amounts of digestible lysine in diet (as-fed basis; 90% dry matter) are 1.12 and 1.02% for male and female chickens, respectively.
c Patterns of amino acid composition in the ideal protein are the same for male and female chickens. The amounts of digestible lysine in diet (as-fed basis; 90% dry matter) are 0.89 and 0.84% for male and female chickens, respectively.
d Patterns of amino acid composition in the ideal protein are the same for male and female chickens. The amounts of digestible lysine in diet (as-fed basis; 90% dry matter) are 0.76 and 0.73% for male and female chickens, respectively.
eThese ratios are based on total amino acids in a typical corn- and soybean meal-based diet.45 The amount of digestible lysine in diet (as-fed basis; 90% dry matter) is 1.2% for 0–21-day-old chicks.
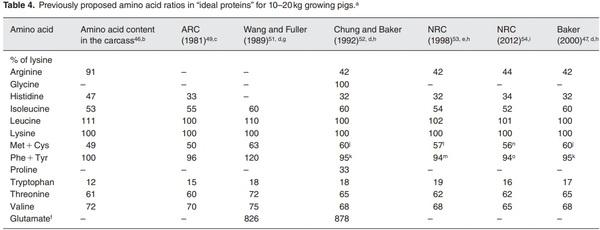
b The body proteins in 20–45kg pigs contain 63g lysine/16g nitrogen.
c These ratios are based on total amino acids in the diet. The total level of lysine in the diet is 1.10% (as-fed basis; 90% dry matter).
d The diet contains 1.20% true digestible lysine (as-fed basis; 90% dry matter).
e The diet contains 1.01% true digestible lysine (as-fed basis; 90% dry matter).
f Provided as the nitrogenous source for synthesis of other NEAA in animals.
g Dietary requirements are for 25–50kg gilts.
h Dietary requirements are for 10–20kg pigs.
i Total amino acid in a typical corn- and soybean meal-based diet (as-fed basis; 90% dry matter). The diet contains 1.40% total lysine.54
j The ratio of l-methionine to l-cystine is 1:1.
kThe ratio of l-phenylalanine to l-tyrosine is 53:47.
l The ratio of l-methionine to l-cystine is 47:53.
m The ratio of l-phenylalanine to l-tyrosine is 64:36.
n The ratio of l-methionine to l-cystine is 51:49.
o The ratio of l-phenylalanine to l-tyrosine is 63:37
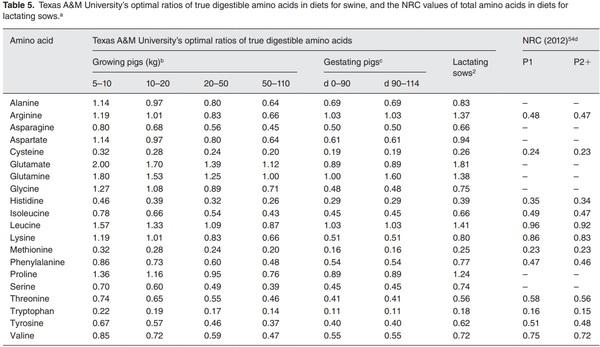
b Fed ad libitum (90% dry matter).
c Fed 2kg/day on days 0–90 and 2.3kg/day on days 90–114 (90% dry matter).
d Data from National Research Council (NRC 2012) refer to total amino acids in a typical corn- and soybean meal-based diet.54 Dry matter content of the diet is 90%. P1=parity 1; P2+=parity 2 or greater.
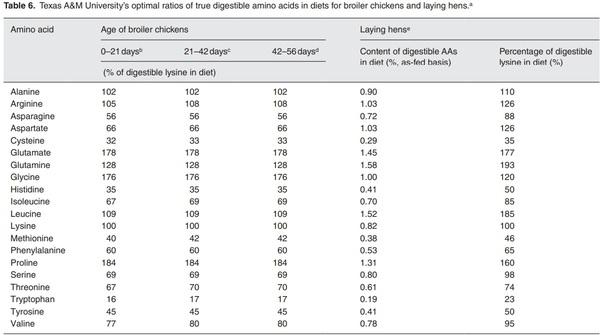
a Except for glycine, all amino acids are l-isomers. Values are based on true ileal digestible amino acids.
b Patterns of amino acid composition in the ideal protein are the same for male and female chickens. The amounts of digestible lysine in diet (as-fed basis; 90% dry matter) are 1.12 and 1.02% for male and female chickens, respectively.
c Patterns of amino acid composition in the ideal protein are the same for male and female chickens. The amounts of digestible lysine in diets (as-fed basis; 90% dry matter) are 0.89 and 0.84% for male and female chickens, respectively.
d Patterns of amino acid composition in the ideal protein are the same for male and female chickens. The amounts of digestible lysine in diets (as-fed basis; 90% dry matter) are 0.76 and 0.73% for male and female chickens, respectively.
e A diet that consists of 60% corn grain (containing 9.3% crude protein) and 24% soybean meal (43.5% crude protein), and is supplemented with 0.2% glycine and 0.1% l-methionine can meet the requirements of laying hens for all amino acids.
1. Phang JM. Perspectives, past, present and future: the proline cycle/ prolinecollagen regulatory axis. Amino Acids 2021;53:1967–75
2. Wu G. Amino acids: biochemistry and nutrition. Boca Raton, FL: CRC
Press, 2022
3. Li XY, Zheng SX, Wu G. Nutrition and functions of amino acids in fish.
Adv Exp Med Biol 2021;1285:133–68
4. Li P, Wu G. Roles of dietary glycine, proline and hydroxyproline in collagen synthesis and animal growth. Amino Acids 2018;50:29–38
5. Moughan PJ, Schultze WH, Smith WC. Amino acid requirements of the growing meat rabbit 1. The amino acid composition of rabbit whole— body tissue—a theoretical estimate of ideal amino acid balance. Anim
Sci 1988;47:297–301
6. Wu ZL, Hou YQ, Dai ZL, Hu CA, Wu G. Metabolism, nutrition and redox signaling of hydroxyproline. Antioxid Redox Signal 2019;30:
674–82
7. Hu SD, He WL, Wu G. Hydroxyproline in animal metabolism, nutrition, and cell signaling. Amino Acids. Epub ahead of print 3 August
2021. DOI: 10.1007/s00726-021-03056-x
8. Bergen WG. Pigs (Sus Scrofa) in biomedical research. Adv Exp Med Biol
2022;1354:335–43
9. Reynolds LP, McLean KJ, McCarthy KL, Diniz WJS, Menezes ACB,
Forcherio JC, Scott RR, Borowicz PP, Ward AK, Dahlen CR, Caton JS.
Nutritional regulation of embryonic survival, growth and development.
Adv Exp Med Biol 2022;1354:63–76
10. Smith BI, Govoni KE. Use of agriculturally important animals as models in biomedical research. Adv Exp Med Biol 2022;1354:315–33
11. Wu G, Wu ZL, Dai ZL, Yang Y, Wang WW, Liu C, Wang B, Wang
JJ, Yin YL. Dietary requirements of “nutritionally nonessential amino acids” by animals and humans. Amino Acids 2013;44:1107–13
12. Davis TA, Fiorotto ML, Reeds PJ. Amino acid compositions of body and milk protein change during the suckling period in rats. J Nutr
1993;123:947–56
13. Li XY, Han T, Zheng SX, Wu G. Nutrition and functions of amino acids in aquatic crustaceans. Adv Exp Med Biol 2021;1285:169–98
14. Boisen S, Hvelplund T, Weisbjerg MR. Ideal amino acid profiles as a basis for feed protein evaluation. Livest Prod Sci 2000;64:239–51
15. Bergen WG. Amino acids in beef cattle nutrition and production. Adv
Exp Med Biol 2021;1285:29–42
16. Cao Y, Yao J, Sun X, Liu S, Martin GB. Amino acids in the nutrition and production of sheep and goats. Adv Exp Med Biol 2021;1285:63–79
17. Mansilla WD, Htoo JK, de Lange CFM. Nitrogen from ammonia is as efficient as that from free amino acids or protein for improving growth performance of pigs fed diets deficient in nonessential amino acid nitrogen. J Anim Sci 2017;95:3093–102
18. Lardy HA, Feldott G. The net utilization of ammonium nitrogen by the growing rat. J Biol Chem 1950;186:509–10
19. Featherston WR, Bird HR, Harper AE. Effectiveness of urea and ammonium nitrogen for the synthesis of dispensable amino acids by the chick. J Nutr 1962;78:198–206
20. Grimson RE, Bowland JP. Urea as a nitrogen source for pigs fed diets supplemented with lysine and methionine. J Anim Sci 1971;33:58–63
21. Rose W, Smith L, Womack M, Shane M. The utilization of the nitrogen of ammonium salts, urea, and certain other compounds in the synthesis of non-essential amino acids in vivo. J Biol Chem 1949;181:
307–16
22. El Boushy AR. Broiler growth response from practical low-protein diets supplemented with urea and diammonium hydrogen phosphate.
Neth J Agric Sci 1980;28:147–55
23. Abderhalden E. Experiment on the feeding with completely degraded nutrition substances. Z Physiol Chem 1912;77:22–58
24. Hou YQ, Yin YL, Wu G. Dietary essentiality of “nutritionally nonessential amino acids” for animals and humans. Exp Biol Med 2015;240:
997–1007
25. Wu G, Bazer FW, Johnson GA, Hou YQ. Arginine nutrition and metabolism in growing, gestating and lactating swine. J Anim Sci 2018;
96:5035–51
26. He WL, Li P, Wu G. Amino acid nutrition and metabolism in chickens.
Adv Exp Med Biol 2021;1285:109–31
27. Wu G, Morris SM Jr. Arginine metabolism: nitric oxide and beyond.
Biochem J 1998;336:1–17
28. Wu G, Bazer FW, Dai ZL, Li DF, Wang JJ, Wu ZL. Amino acid nutrition in animals: protein synthesis and beyond. Annu Rev Anim Biosci
2014;2:387–417
29. He WL, Furukawa K, Toyomizu M, Nochi T, Bailey CA, Wu G. Interorgan metabolism, nutritional impacts, and safety of dietary L-glutamate and L-glutamine in poultry. Adv Exp Med Biol 2021;1332:107–28
30. Hou YQ, Wu G. Nutritionally nonessential amino acids: a misnomer in nutritional sciences. Adv Nutr 2017;8:137–9
31. Herring CM, Bazer FW, Wu G. Amino acid nutrition for optimum growth, development, reproduction, and health of zoo animals. Adv
Exp Med Biol 2021;1285:233–53
32. Glista WA, Mitchell HH, Scott HM. The amino acid requirements of the chick. Poult Sci 1951;30:915
33. Fisher H, Scott HM. The essential amino acid requirements of chicks as related to their proportional occurrence in the fat-free carcass. Arch
Biochem Biophys 1954;51:517–9
34. Klain GJ, Scott HM, Johnson BC. The amino acid requirements of the growing chick fed a crystalline amino acid diet. Poultry Sci 1960;39:
39–44
35. Baker DH, Sugahara M, Scott HM. The glycine-serine interrelationship in chick nutrition. Poult Sci 1968;47:1376–7
36. Graber G, Baker DH. The essential nature of glycine and proline for growing chickens. Poult Sci 1973;52:892–6
37. Baker DH. Advances in protein-amino acid nutrition of poultry. Amino
Acids 2009;37:29–41
38. Coon CN, Grossie VB Jr, Couch JR. Glycine-serine requirement for chicks. Poult Sci 1974;53:1709–13
39. Dean WF, Scott HM. The development of an amino acid reference diet for the early growth of chicks. Poult Sci 1965;44:803–8
40. Huston RL, Scott HM. Effect of varying the composition of crystalline amino acid mixture on weight gain and pattern of free amino acids in chick tissue. Fed Proc 1968;27:1204–9
41. Sasse CE, Baker DH. Modification of the Illinois reference standard amino acid mixture. Poult Sci 1973;52:1970–2
42. Baker DH, Han Y. Ideal amino acid profile for broiler chicks during the first three weeks posthatching. Poult Sci 1994;73:1441–7
43. Price WA Jr, Taylor MW, Russell WC. The retention of essential amino acids by the growing chick. J Nutr 1953;51:413–22
44. Robel E, Menge H. Performance of chicks fed an amino acid profile diet based on carcass composition. Poult Sci 1973;52:1219–21
45. National Research Council (NRC). Nutrient Requirements of Poultry, ninth revised edition. Washington DC: National Academy Press, 1994
46. Baker DH. Ideal amino acid profiles for swine and poultry and their applications in feed formulation. BioKyowa Tech Rev 1997;9:1–24
47. Baker DH. Recent advances in use of the ideal protein concept for swine feed formulation. Asian-Aus J Anim Sci 2000;13:294–301
48. Cole DJA. The amino acid requirements of pigs: the concept of an ideal protein. Pig News Info 1980;1:201–5
49. Agricultural Research Council (ARC). The nutrient requirements of pigs: technical review. Slough: Commonwealth Agricultural Bureaux, 1981
50. National Research Council (NRC). Nutrient requirements of swine (9th edn). Washington DC: National Academy Press, 1988
51. Wang TC, Fuller MF. The optimum dietary amino acid patterns for growing pigs. 1. Experiments by amino acid deletion. Br J Nutr 1989;62:77–89
52. Chung TK, Baker DH. Ideal amino acid pattern for ten kilogram pigs.
J Anim Sci 1992;70:3102–11
53. National Research Council (NRC). Nutrient requirements of swine (10th edn). Washington, DC: National Academy Press, 1998
54. National Research Council (NRC). Nutrient requirements of swine (11th edn). Washington, DC: National Academy Press, 2012
55. Rezaei R, Knabe DA, Tekwe CD, Dahanayaka S, Ficken MD, Fielder
SE, Eide SJ, Lovering SL, Wu G. Dietary supplementation with monosodium glutamate is safe and improves growth performance in postweaning pigs. Amino Acids 2013;44:911–23
56. Wu G, Bazer FW, Johnson GA, Knabe DA, Burghardt RC, Spencer TE,
Li XL, Wang JJ. Important roles for L-glutamine in swine nutrition and production. J Anim Sci 2011;89:2017–30
57. Wang WW, Dai ZL, Wu ZL, Lin G, Jia SC, Hu SD, Dahanayaka S, Wu
G. Glycine is a nutritionally essential amino acid for maximal growth of milk-fed young pigs. Amino Acids 2014;46:2037–45
58. van Milgen J, Dourmad JY. Concept and application of ideal protein for pigs. J Anim Sci Biotechnol 2015;6:15
59. Kim SW, Wu G, Baker DH. Ideal protein and amino acid requirements by gestating and lactating sows. Pig News Inform 2005;26:89N–99N
60. Ekmay RD, De Beer M, Mei SJ, Manangi M, Coon CN. Amino acid requirements of broiler breeders at peak production for egg mass, body weight, and fertility. Poult Sci 2013;92:992–1006
61. Kim SW, Hurley WL, Wu G, Ji F. Ideal amino acid balance for sows during gestation and lactation. J Anim Sci 2009;87:E123–32
62. Eugenio FA, van Milgen J, Duperray J, Sergheraert R, Le Floc’h N.
Feeding intact proteins, peptides, or free amino acids to monogastric farm animals. Amino Acids 2022;54:157–68
63. National Research Council (NRC). Nutrient requirements of fish and shrimp. Washington, DC: National Academies Press, 2011
64. Dierenfeld ES, Wedekind KJ, Middelbos I. Ideal protein and zoo carnivores: further considerations for optimizing diets. In: Proceedings of the ninth conference on zoo and wildlife nutrition, AZA Nutrition Advisory
Group (ed A Ward, A Coslik, M Maslanka M), Kansas City, MO, 22–28,
October, 2011, pp.1–5
65. Frantz NZ, Yamka RM, Friesen KG. The effect of diet and lysine: calorie ratio on body composition and kidney health in geriatric cats. Intern
J Appl Res Vet Med 2007;5:25–36
66. Baker DH. Comparative nutrition of cats and dogs. Annu Rev Nutr
1991;11:239–63
67. Wu G. Dietary requirements of synthesizable amino acids by animals: a paradigm shift in protein nutrition. J Anim Sci Biotechnol 2014;5:34
68. Hou YQ, Wu G. L-Glutamate nutrition and metabolism in swine.
Amino Acids 2018;50:1497–510
69. Zhang Q, Hou YQ, Bazer FW, He WL, Posey EA, Wu G. Amino acids in swine nutrition and production. Adv Exp Med Biol 2021;1285:81–107
70. Zhu C, Li XL, Bazer FW, Johnson GA, Burghardt RC, Jiang ZY, Wu
G. Dietary L-arginine supplementation during days 14-25 of gestation enhances aquaporin expression in the placentae and endometria of gestating gilts. Amino Acids 2021;53:1287–95
71. Elmetwally MA, Li XL, Johnson GA, Burghardt RC, Herring CM,
Kramer AC, Meininger CJ, Bazer FW, Wu G. Dietary supplementation with L-arginine between Days 14 and 25 of gestation enhances NO and polyamine syntheses and expression of angiogenic proteins in porcine placentae. Amino Acids 2022;54:193–204
72. Jia SC, Li XY, Wu G. The lack of dietary glutamate and/or glutamine is detrimental to juvenile hybrid striped bass. In: Aquaculture America annual meeting, New Orleans, LA, 7–11 March 2019
73. Homma C, Hirose K, Ito T, Kamikawa M, Toma S, Nikaido S, Satoh
M, Uemoto Y. Estimation of genetic parameter for feed efficiency and resilience traits in three pig breeds. Animal 2021;15:100384
74. Patience JF, Rossoni-Serao MC, Gutierrez NA. A review of feed efficiency in swine: biology and application. J Anim Sci Biotechnol 2015; 6:33–42
75. Maharjan P, Martinez DA, Weil J, Suesuttajit N, Umberson C,
Mullenix G, Hilton KM, Beitia A, Coon CN. Physiological growth trend of current meat broilers and dietary protein and energy management approaches for sustainable broiler production. Animal 2021;15:100284
76. Deng D, Li AK, Chu WY, Huang RL, Li TJ, Kong XF, Liu ZJ, Wu GY,
Zhang YM, Yin YL. Growth performance and metabolic responses in barrows fed low-protein diets supplemented with essential amino acids. Livest Sci 2007;109:224–7
77. Peng X, Hu L, Liu Y, Yan C, Fang ZF, Lin Y, Xu SY, Li J, Wu CM, Chen
DW, Sun H, Wu D, Che LQ. Effects of low-protein diets supplemented with indispensable amino acids on growth performance, intestinal morphology and immunological parameters in 13 to 35 kg pigs. Animal
2016;10:1812–20
78. Kidd MT, Maynard CW, Mullenix GJ. Progress of amino acid nutrition for diet protein reduction in poultry. J Anim Sci Biotechnol 2021;12:45
79. Liu SY, Macelline SP, Chrystal1 PV, Selle PH. Progress towards reduced-crude protein diets for broiler chickens and sustainable chicken-meat production. J Anim Sci Biotechnol 2021;12:20
80. Chalvon-Demersay T, Luise D, Le Floc’h N, Tesseraud S, Lambert
W, Bosi P, Trevisi P, Beaumont M, Corrent E. Functional amino acids in pigs and chickens: implication for gut health. Front Vet Sci
2021;8:663727
81. Wu G. Amino acids in nutrition, health, and disease. Front Biosci 2021;
26:1386–92
82. Li XL, Johnson GA, Zhou HJ, Burghardt RC, Bazer FW, Wu G. Microarray analysis reveals an important role for dietary L-arginine in regulating global gene expression in porcine placentae during early gestation.
Front Biosci 2022;27:033
83. Che DS, Nyingwa PS, Ralinala KM, Maswanganye GMT, Wu G.
Amino acids in the nutrition, metabolism, and health of domestic cats.
Adv Exp Med Biol 2021;1285:217–31
84. Li P, Wu G. Composition of amino acids and related nitrogenous nutrients in feedstuffs for animal diets. Amino Acids 2020;52:523–42
85. Le Floc’h N, Wessels A, Corrent E, Wu G, Bosi P. The relevance of functional amino acids to support the health of growing pigs. Anim Feed Sci
Technol 2018;245:104–16
86. Belloir P, Méda B, Lambert W, Corrent E, Juin H, Lessire M, Tesseraud
S. Reducing the CP content in broiler feeds: impact on animal performance, meat quality and nitrogen utilization. Animal 2017;11:1881–9
87. Belloir P, Lessire M, Lambert W, Corrent E, Berri C, Tesseraud S.
Changes in body composition and meat quality in response to dietary amino acid provision in finishing broilers. Animal 2019;13:1094–102
88. Li XL, Zheng SX, Wu G. Nutrition and metabolism of glutamate and glutamine in fish. Amino Acids 2020;52:671–91
89. Chrystal PV, Moss AF, Khoddami A, Naranjo VD, Selle PH, Liu SY.
Impacts of reduced-crude protein diets on key parameters in male broiler chickens offered maize-based diets. Poult Sci 2020;99:505–16
90. Wu G. Nutrition and metabolism: foundations for animal growth, development, reproduction, and health. Adv Exp Med Biol 2022;1354:
1–24
91. Wang Y, Zhou J, Wang G, Cai S, Zeng XF, Qiao SY. Advances in lowprotein diets for swine. J Animal Sci Biotechnol 2018;9:60
92. Institute of Medicine (IOM). Dietary reference intakes for energy, carbohydrates, fiber, fat, fatty acids, cholesterol, proteins, and amino acids. Washington,
DC: The National Academies Press, 2005




I congratulate the authors for the information contained in this article, which I fully agree with. I also understand that the article that was published on ENGORMIX, under the title Digestible lysine levels obtained by two methods of formulation of diets for 22-to-42-day-old broilers, under my guidance, is somehow consistent with the information described here.

can some one explain what is ideal protein concept?
Very interesting and I will be attentive to the discussion which should be very enriching.


















