One of our most effective strategies to combat bacterial infections is rapidly becoming ineffective: more and more bacteria are developing resistance against commonly used antibiotics. This is a worrysome development, that, though been recognized for decades, seems to have worsened lately. It is perceived that the speed of resistance development has overhauled the rate of developing alternative drugs.
In attempts to stop this unwanted process, the use of antibiotics in veterinary medicine has become a focus of attention, especially in view of the broad range of drugs that are in use in both disciplines. The reasoning is that animals receiving antibiotics can also carry bacteria that are human pathogens (zoonoses), which develop resistance during treatment of the animal. When these resistant bacteria subsequently cause infections in patients these are hard to treat as a result of the resistance. This consequences to public health could be an increase in disease severity, complications, and even in mortality. This seemingly reasonable scenario has resulted in a demand to restrict the use of clinically relevant antibiotics in veterinary medicine. Here we would like to test the validity of the arguments used in the discussion by means of two selected examples. Further we would like to dig a bit deeper in the processes that result in resistance, and what can be done in a practical setting to prevent these.
Campylobacter is our first example, since it is a common zoonotic pathogen that is mainly transmitted through food of animal origin. In many countries it is a more frequent cause of human diarrhoea than Salmonella. Campylobacteriosis is most often self-limiting, and only requires antibiotic treatment in patients at risk, or in severe cases. Up to 100% of poultry flocks can be found positive for C. jejuni. One single point mutation in the bacterial gene coding for gyrase is all that is needed for Campylobacter to become resistant to clinical concentrations of fluoroquinolones (Fq). The use of Fq in poultry husbandry results in a selection of resistant bacteria, but it should be noted that selection takes place when Fq is used in humans, too. Since human-to-human transmission is rare, a patient can be considered a 'dead-end host' for this pathogen. Transmission from poultry meat to humans is one of the most common sources of infection.
Since the use of Fq was introduced in both human and veterinary medicine, the frequency of Fq-resistence (FqR) in Campylobacter has rapidly increased. As a consequence, camylobacteriosis is not effectively treateble with Fq and erythromycin is now the first-choice antibioticum. The most common complication, Guillain-Barré-Syndrom (an autoimmune disease), is independent of antibiotic resistance. Countries have reported different frequencies of FqR in their Campylobacter populations but this doesn't correlate with the relative frequency of clinical complications or mortality; these have also not increased in the past 15 years since FqR in Campylobacter is on the rise.
Case-control studies have reported that campylobacteriosis cases resulting from FqR infections were either more severe, or lasted longer, than infections with susceptible bacteria, but others who re-examined these data came to the conclusion that there was no difference (Wassenaar et al, 2007), and that conclusion was backed up by another, larger, epidemiological study (anonymous, 2002). Thus, although it is true that the use of Fq in poultry has resulted in development of resistance in Campylobacter, the impact of this resistance to human health is limited.
The second example deals with Salmonella in poultry, and here it is important to note that human salmonellosis is frequently treated with Fq, where the drug is shown to be effective (even though the majority of salmonellosis cases are self-limited and do not require antibiotic treatment). So far we have only observed a partial resistance (reduced susceptibility) against Fq in Salmonella isolated from poultry. These bacteria are still likely to respond to Fq treatment in a human patient, provided the antibiotic is used at the correct dose. Complete resistance has been established in the laboratory, and was observed in a few cases human isolates, but complete resistant Salmonella have so far not been isolated from animals. It may be that the complete resistance has a cost in fitness of the bacteria to survive and multiply in animals. It is therefore not correct to attribute the serious consequences of potentially life-threatening, though rare, complete Fq resistance in Salmonella to the use of Fq in the poultry industry.
As the two cases mentioned above illustrate, there is a disconnect in the argumentation: although the use of a specific antibiotic in veterinary medicine can result in resistance of zoonotic bacteria, it is not possible to attribute treatment failure in humans to these resistances. Zoonotic bacteria such as E. coli, Campylobacter, Salmonella, all show a complex epidemiology where particular serotypes, resistence types, Pathotypes and host specificity have to be taken into account, playing different roles depending on the species. It is impossible to assess the relative fraction of resistances that result from veterinary antibiotic use and separate these from consequences of human use (Wassenaar, 2005). Veterinary medicine is, however, fully responsible for resistances that are encountered in pathogens that exclusively or mainly infect animals, such as in animal isolates of M. avium paratuberculosis, Mycoplasma bovis, Streptococcus suis, Pasteurella multocida or Mannheimia haemolytica. Interestingly, for some of these pathogens, antibiotic resistance is not a major issue.
We must conclude that it is easy to blame veterinary use of antibiotics for resistances that produce untreatable human infections, but that it is impossible to give evidence that this blame is justified. Some would argue that 'absence of evidence' is not the same as 'evidence of absence', and that the precautionary principle would urge us to restrict the use of antibiotics in animals when these are of major importance in human treatment. Naturally, antibiotics should always be used with care, and only in cases where its use is justified. That applies to human as well as to animal treatment.
The conclusion of the above examples should not be that resistance in pathogenic bacteria is of no concern to human health. We experience a large number of serious pathogens becoming rapidly resistant to commonly used antibiotics, and the consequences of this development are severe.The importance of veterinary medicine in the selection of problematic resistances is relatively minor compared to the resistance problems encountered with nosocomial (hospital) infections or the resistances observed in exclusively human pathogens. For example, consider the serious multiple resistances in in Helicobacter pylori, Mycobacterium tuberculosis, or Neisseria gonorrhoe. For all these pathogens a serious increase in resistance causes acute problems, whereby multiple drug therapy or novel drugs provide only temporary solutions to treat their infections (Wassenaar & Silley, 2008).
The efforts to look for a veterinary source of resistances can have undesired consequences. Following detection of methicillin-resistant Staphylococcus aureus on fattening pigs, pork samples are now being checked for presence of MRSA. Though these are still research investigations, positive findings may eventually urge detection on a routine basis. Since life-threatening MRSA infections are mostly nosocomial and rarely result of foodborne contamination, any money spent on detection of MRSA in food could better be spent to avoid contamination and spread in hospitals. Likewise, concentrating our efforts on removal of resistant zoonotic bacteria from the food chain would be folly, as food safety is only improved when all zoonotic bacteria are being kept in check, whether they are resistent or not.
Our efforts to reduce resistance should concentrate on those occasions where these bacteria are selected for resistance, which is when they encounte antibiotics in whatever host. Let's consider what happens to a bacterial population when an antibiotic is present. Resistance can result from single-point mutations or gene uptake, events that can take place any time. Therefore, we assume that in any bacterial population some cells are present that are resistant against a given antibiotic compound, even though the complete population would still be reported as 'susceptible'. This simply means that the vast majority of the cells present are susceptable. An acute infection without antibiotic treatment would not change this minute proportion of resistant cells, as shown in the figure. When, however, antibiotics are being applied, this will for those cells that are resistant, and the longer the drug is present, the larger this fraction becomes, untill all remaining cells are resistant: the population has 'developed resistance'.
It is a myth that short therapy will result in resistance and that longer treatment can prevent this. Quite in contrast, a therapy that is used longer than necessary will remove all susceptible bacteria (after all the resistant ones are, well, resistant). In case the treatment was stopped before this, some susceptible cells would remain present, and these could, without selective pressure (in another animal, in the environment, in another patient who is not taking the same drug), outgrow the resistant population. This route back towards increases susceptibility, of the bacterial population as a whole, is blocked when long treatment has effectively eliminated all susceptible organisms. A new mutation would be needed to reduce resistance in that case.
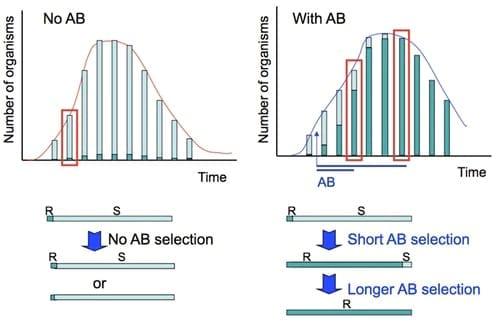
Figure. To the left is indicated in a schematic way how a self-limiting acute infection would progress without any antibiotic treatment. We assume that a minor population of resistant cells is already present at the onset of infection, and their proportion remains constant over time. Below the graph, the proportion of resistant cells in the population before and after treatment is shown. To the right is shown what happens if an antibiotic is given, either for a short or for a longer period: resistant bacteria are selected. This selection can be complete when therapy is extended over time.
Modern insights dictate that it is better to treat an acute infection short rather than long. Many scientific publications describe nowadays how short a therapy can be to be still effective (e.g. Rubinstein, 2007). These novel treatment regimes should be applied in medical practices now, as they can stop the resistance problem from going ever worse. Treatment duration should depend on the pathogen and type of infection, and should not be dictated by the size of the package. As a rule of thumb, an antibiotic can be stopped when a patient has been free of fever or symptoms for a certain period. This is already being practiced in hospitals, and should be advised by general practicioners treating the community as well. When the choice is between a longer treatment with a lower dose, or a shorter treatment with a higher dose, the latter is to be preferred if resistances are to be avoided, provided that side effects remain manageable. Long-term therapy and the increase of the dose during treatment should be avoided whenever possible.
In conclusion, the veterinary and human medicine can both contribute to reduce the problem of resistance, when they keep the use of antibotics as minimal as possible. When needed, an antibiotic should be used for a short time only, and be applied at the correct dose.
Literature:
Campylobacter Sentinel Surveillance Scheme Collaborators. (2002). Ciprofloxacin resistance in Campylobacter jejuni: case-case analysis as a tool for elucidating risks at home and abroad. J Antimicrob Chemother. 50: 561-568
Wassenaar TM. (2005). Use of antimicrobial agents in veterinary medicine and implications for human health. Critical Rev. Microbiob. 31: 155-169.
Wassenaar TM. and Silley P. (2008). Antimicrobial resistance in zoonotic bacteria: lessons learned from host-specific pathogens. Anim Health Res Rev. 9: 177-186.
Wassenaar, TM., Kist, M. and de Jong, A. (2007) Re-analysis of the risks attributed to ciprofloxacin-resistant Campylobacter jejuni infections. Int J Antimicrob Agents 30: 195-201.
Rubinstein E. (2007). Short antibiotic treatment courses or how short is short? Int J Antimicrob Agents S1:76-79.






















