INTRODUCTION
Phytase is commonly supplemented in laying hen diets to increase the bioavailability of P and Ca. Dietary phytase supplementation increases Ca and P digestibility (Beutler, 2009), egg production, BW, eggshell quality, and bone quality in egg-laying hens (Hughes et al., 2009; Pelicia et al., 2009) and increases blood myo-inositol concentration in broilers (Sommerfeld et al., 2018).
The effects of phytase in diets reduced in available P (avP) and Ca on laying hen bone quality are mainly reported in terms of bone breaking strength and ash content (Boling et al., 2000; de Lima et al., 2010). Bones, particularly long bones, in egg-laying hens are comprised of cortical, trabecular, and medullary bone tissues. Cortical and trabecular tissues are the strength-providing structural bone (Reich and Gefen, 2006), whereas medullary bone is a nonstructural tissue which stores mineral to support egg-shell formation (Fleming et al., 1998). In actively laying hens, the cortical and trabecular bone tissues are not formed but may be resorbed, whereas the medullary bone tissue is resorbed to support eggshell formation but re-deposited when a shell is not being calcified (Dacke et al., 1993). Over the laying cycle, the strength-providing cortical and trabecular bone tissues will gradually decrease, whereas medullary bone can gradually accumulate (Fleming et al., 1998). Hence, changes in bone ash as a result of experimental treatment may have little to no relationship with laying hen bone resistance to breakage. Bone breaking strength (BBS) is assessed with the application of a perpendicular force on the mid-diaphysis of a long bone, a site primarily concentrated with cortical and medullary bone tissues (de Lima et al., 2010; Kerschnitzki et al., 2014). However, the proximal and distal ends of long bones have less of the compact cortical bone but more spongy trabecular bone (Reich and Gefen, 2006). The bone metaphysis is more susceptible to fracture than the mid-diaphysis, which is usually the location of bone breaking strength testing (Reich and Gefen, 2006). Although BBS provides information on fracture resistance specific to the mid-diaphysis (de Lima et al., 2010; Regmi et al., 2015), the assessment does not provide information on metaphyses, the bone regions that are most susceptible to fracture (Reich and Gefen, 2006). Overall, BBS and bone ash, P, and Ca contents are commonly used to measure bone quality as they are easy, fast, and inexpensive to measure relative to bone imaging technologies such as quantitative computed tomography (QCT). Therefore, although bone ash and BBS are important parameters that provide information on bone mineralization and resistance to fracture, respectively, it is vital to interpret the results in light of the limitations of these measures.
Densitometry of each specific bone fraction and assessment of the circulating concentrations of bone biochemical markers can help to understand the effects of dietary phytase on bone biology. Quantitative computed tomography has been used to evaluate bone mass and mineralization in different bone tissue fractions in egg-laying hens (Saunders-Blades et al., 2009; Kim et al., 2011; Casey-Trott et al., 2017a,b). Serum osteocalcin (bone formation marker), pyridinoline (bone resorption marker), and parathyroid hormone (PTH), which plays an important role in blood P and Ca homeostasis, and parameters such as serum Ca and P have been used to assess bone formation and resorption in hens (Jiang et al., 2013) and pullets (Regmi et al., 2015). However, little information is available on the effects of dietary Ca and avP levels and phytase supplementation on bone metabolism in laying hens. It was hypothesized that phytase supplementation would alleviate the adverse effects of moderate and severe reductions in dietary avP and Ca on performance, bone densitometry, and serum biochemical markers of bone metabolism in laying hens. The objective of this study was to evaluate the effects of moderate and severe reductions of dietary Ca and avP and phytase supplementation on bone densitometry; serum osteocalcin, pyridinoline, PTH, Ca, and P concentrations.
MATERIALS AND METHODS
The Animal Care and Use Committee: Livestock of the University of Alberta approved the protocol of the current study, and the animal handling procedure followed was consistent with the Canadian Council on Animal Care guidelines (CCAC, 2009). One hundred H&N Nick Chick Single-comb White Leghorn hens were obtained at 68 wk of age (woa) from a flock of healthy laying hens at the Poultry Research Centre of the University of Alberta. The hens were housed individually (48 x 43 x 41 cm for width, depth, and height, respectively; Specht Canada Inc., Stony Plain, AB, Canada) in a double-tier cage system located in an environmentally controlled facility. The hens were fed a typical commercial laying hen diet adequate in avP and Ca from 66 to 68 woa. From that group, 100 hens with at least 93% egg production during the 2-wk pre-experimental period were selected for the trial. The hens were managed as recommended by the primary breeder (H&N International, 2016).
Treatments
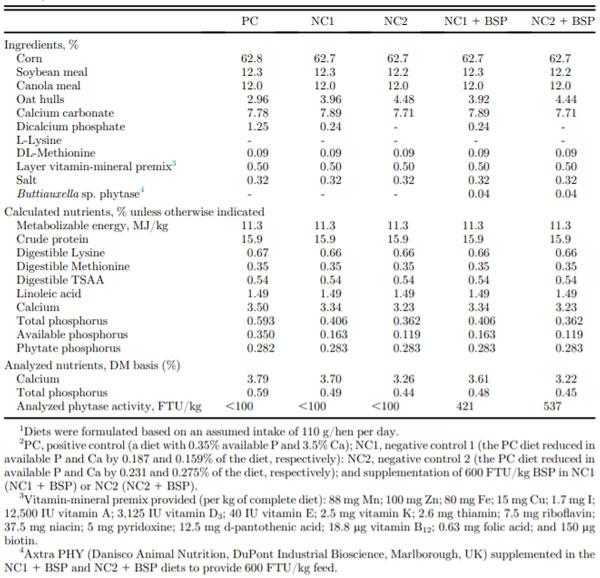
Each of 5 dietary treatments was randomly assigned to 20 individually housed hens from 68 to 78 woa. The mash diets included a positive control (PC) with 0.35% avP and 3.5% Ca, and the PC with avP and Ca levels moderately reduced by 0.187 and 0.159% of the diet, respectively (NC1; 53 and 4.5% less than the PC, respectively) or severely reduced by 0.231 and 0.275% of the diet, respectively (NC2; 66 and 7.9% less than the PC, respectively). Other diets were the NC1 or NC2 diets supplemented with Buttiauxella sp. phytase (BSP) at 600 FTU/kg (NC1 + BSP or NC2 + BSP, respectively). Previously, marginal reductions of avP (by 0.146% of the diet) and Ca (by 0.134% of the diet) in commercial laying hen diets did not decrease egg production and eggshell quality when fed from 30 to 70 woa (Bello and Korver, 2019). Hence, the moderate reductions in dietary avP by 0.187% of the diet and Ca 0.159% of the diet in the NC1 treatment were based on the expected P- and Ca-releasing activity of the supplemented BSP at 600 FTU/kg. The severe reduction in dietary avP (by 0.231% of the diet) and Ca (by 0.275% of the diet) in the NC2 diet were greater than the levels expected to be released by 600 FTU/kg BSP; the rationale was to assess the efficacy of BSP at 600 FTU/kg of diet with a more substantial reduction of dietary avP and Ca. The formulated ingredients, calculated nutrient composition, and analyzed Ca and total P levels, and phytase activity in diets are shown in Table 1. The phytase used in the study was AxtraPHY (Danisco Animal Nutrition, DuPont Industrial Biosciences, Marlborough, UK), which was sourced from Buttiauxella sp. and expressed in Trichoderma reesei. The phytase activity in each of the diets was analyzed using Method 30,024 of ISO, (2009).
Performance and Eggshell Quality
Egg production was assessed daily and calculated on a weekly basis. On a 2-wk basis, individual hen BW and average daily feed intake were determined, and feed conversion ratio (FCR) was calculated for each hen based on kg of feed consumed per dozen eggs produced. Also, at each of 68, 70, 72, 74, 76, and 78 woa, eggshell breaking strength and specific gravity of all eggs produced on those days were determined as described by Bello and Korver (2019).
Table 1. Dietary ingredient and calculated nutrient content and analyzed Ca and P levels and phytase activity.1,2
Serum Collection and Bone Biochemical Analyses
At 78 woa, 12 hens per treatment (n = 60) were bled between 7:00 am and 11:30 am. Serum was separated from the blood (Jiang et al., 2013) and stored at −80 °C until the time of analyses. The serum samples were subsequently thawed and analyzed for osteocalcin and pyridinoline using commercial ELISA kits (Quidel Corporation, San Diego, CA), with procedures described by the manufacturer. The commercial ELISA kits used for the osteocalcin and pyridinoline have been used in assessment of bone formation and resorption indicators, respectively (Regmi et al., 2015). For PTH, a chicken-specific ELISA kit (Cusabio, Baltimore, MD) was used following the manufacturer’s recommended procedures. Also, serum Ca and P levels were analyzed as described by Bello and Korver (2019).
Bone Quality Sample Collection and Analyses
At 78 woa, all hens were euthanized by cervical dislocation, and the left femurs were excised for subsequent bone densitometry assessment using QCT. The femurs (n = 100) were analyzed at the mid-diaphysis (at 50% of bone length) and proximal metaphysis (30% from the proximal end) for total, cortical, and trabecular space bone mineral densities (BMD; mg/cm3) and cross-sectional areas (BCSA; mm2). Bone mineral content (BMC; mg/mm) was calculated by multiplying BMD by BCSA to determine the amount of mineral (mg) contained within a 1 mm-thick slice perpendicular to the bone length (Korver et al., 2004; Saunders-Blades et al., 2009; Bello and Korver, 2019).
Experimental Design and Statistical Analysis
The individually housed hen was the experimental unit in a completely randomized experimental design. The egg production data were not normally distributed and were log transformed before analysis. However, treatment means for the egg production analysis was presented as the original values. The MIXED procedure of SAS 9.3 (SAS, 2013) was used for analysis of the log-transformed egg production, feed intake, FCR, BW, and eggshell breaking strength and specific gravity in repeated measure analysis for the diet x age interaction and diet and age main effects. In the repeated measure analysis for each of the parameters, the fixed variables (diet x age interaction and diet and age main effects) were analyzed with age as the repeated subject. Also, the MIXED procedure of SAS 9.3 (2013) was used for analysis of diet main effect for each of the biochemical bone markers and bone densitometry parameters. When significant, BW was included in the analyses of the bone densitometry parameters as covariate. Effects were significant when P ≤ 0.05. LSMEANS was used for means comparison.
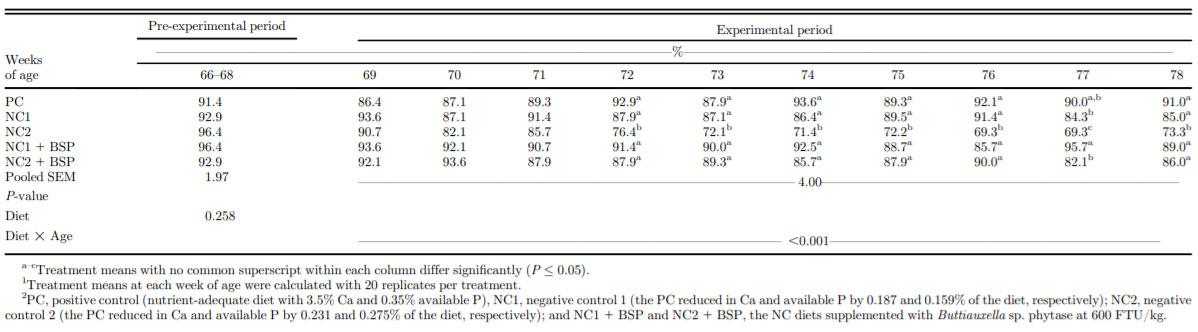
Table 2. Effect of dietary Ca, available P levels, and phytase on % hen-day egg production of single-comb White Leghorn hens from 68 to 78 wk of age.1,2
RESULTS
Performance and Eggshell Quality Parameters
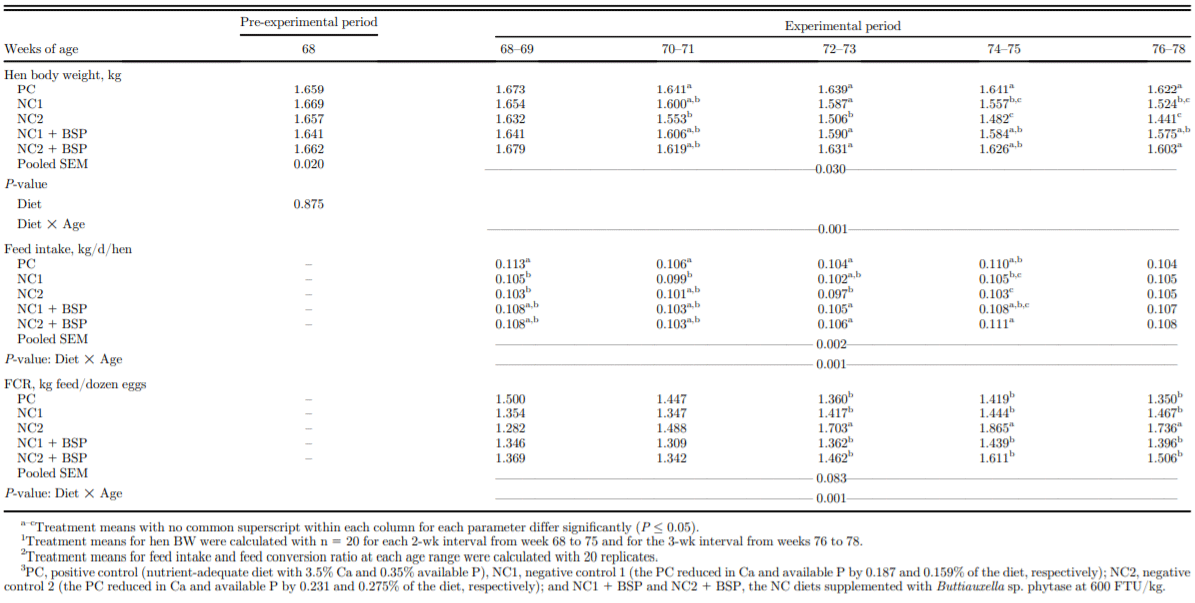
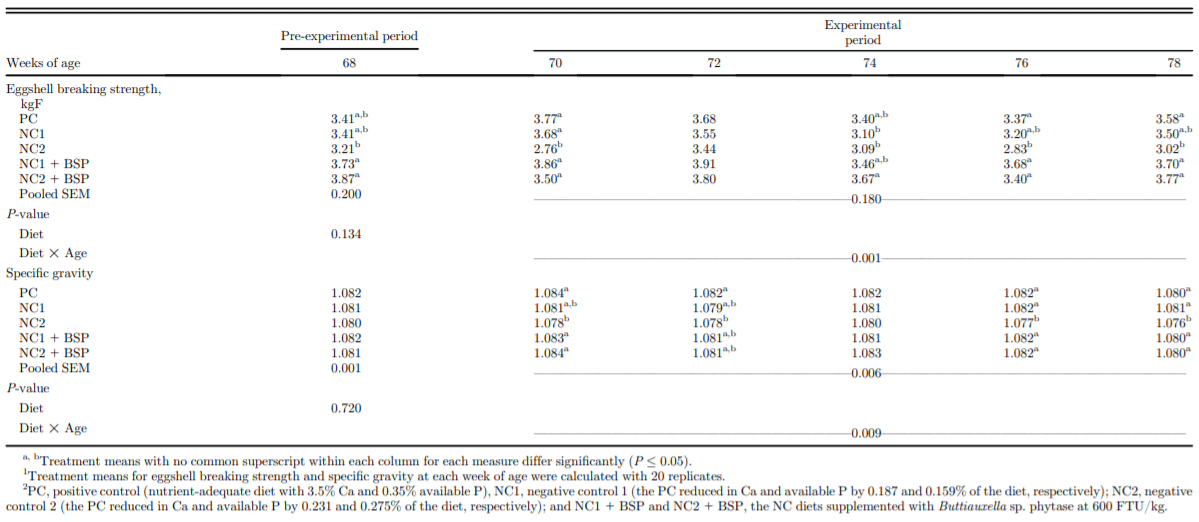
From 68 to 71 woa, egg production was not affected by dietary treatment (Table 2). Egg production was not different between PC, NC1, and NC1 + BSP hens except at 77 woa, when egg production by the NC1 and NC2 hens was increased by BSP supplementation. However, the NC2 diet reduced egg production relative to all other treatments from 72 to 78 woa (P < 0.001). Hen BW was not affected by diet at the start of the experiment (68 woa) nor at 70 woa (P = 0.001; Table 3). BW was decreased by NC1 relative to PC at each of 76 and 78 woa and was intermediate in the NC1 + BSP at both ages. BW of NC2 hens was lower than the PC hens at each of 72, 74, 76, and 78 woa and was intermediate in NC2 + BSP hens at 72 woa. Phytase addition to the NC1 and NC2 diets alleviated the decreased BW of hens relative to the PC diet. Feed intake from 68 to 69 woa was lower in NC1 and NC2 than PC and from 70 to 71 woa was lower in NC1 than in PC and was intermediate in the NC2 and respective BSP-supplemented diets (P = 0.011). Feed intake from 72 to 73 and from 74 to 75 was maintained by NC1 but decreased by NC2 relative to PC and NC2 + BSP, but no differences between diets were observed from 76 to 78 woa. Feed conversion ratio was maintained by NC1 relative to PC and NC1 + BSP throughout the study and was maintained from 68 to 69 and 70 to 71 woa but decreased from 72 to 73, 74 to 75, and 76 to 78 woa by the NC2 relative to the PC and NC2 + BSP (P < 0.001). Eggshell breaking strength was maintained by NC1 and NC2 relative to PC and the respective BSP-supplemented diets at all ages except at 70, 76, and 78 woa, however, was higher in NC2 + BSP than NC2 at 68 woa (P = 0.001; Table 4). At each of 70, 76, and 78 woa, eggshell breaking strength was decreased by the NC2 relative to the PC and NC2 + BSP treatments. Egg-specific gravity was not different between diets at 68 and 74 woa but was lower for NC2 than in PC and NC2 + BSP at 70, 76, and 78 woa (P = 0.009). At 72 woa, NC2 had lower specific gravity than PC but was intermediate in NC2 + BSP.
Table 3. Effect of dietary Ca, available P levels, and phytase on body weight, feed intake, and feed conversion ratio of single-comb White Leghorn hens from 68 to 78 wk of age.1,2,3
Serum Biochemical Bone Markers and Bone Densitometry
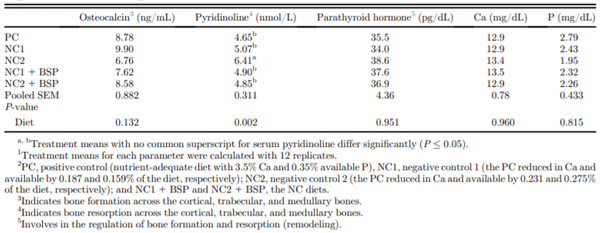
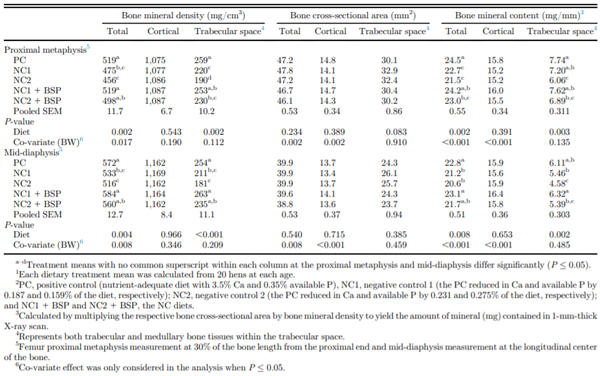
Serum pyridinoline concentration was maintained by NC1 relative to PC and NC1 + BSP and was increased by NC2 relative to the other diets (P = 0.002; Table 5). There was no diet effect on serum osteocalcin, PTH, P, or Ca concentrations. Proximal metaphysis and middiaphysis total BMD and mid-diaphysis trabecular space BMD were each lower (P ≤ 0.002, 0.004, and 0.001, respectively) for NC1 and NC2 than PC, NC1 + BSP, and NC2 + BSP (Table 6). Proximal metaphysis trabecular space BMD was decreased by NC1 and NC2 relative to PC, but supplemental BSP completely alleviated the adverse effect of dietary mineral reductions in the NC1 diet only (P = 0.002). The trabecular space BCSA tended to be higher (P = 0.083) for NC1 and NC2 relative to PC, NC1 + BSP, and NC2 + BSP. The effects of diet for proximal metaphysis and mid-diaphysis total BMC and mid-diaphysis trabecular space BMC were each similar (P = 0.002, 0.008, and 0.002, respectively) to the pattern for trabecular space BMD in the proximal metaphysis. Also, the proximal metaphysis trabecular space BMC was maintained by NC1 and was decreased by NC2 and NC2 + BSP relative to the PC (P = 0.003).
DISCUSSION
Effects of Moderate and Severe Deficiencies of Dietary Available P and Ca
The avP and Ca levels in the NC1 diet maintained performance throughout the 10 wk study but at the expense of BW and bone mineralization. The NC2 diet decreased productivity after 71 woa, but the NC1 diet maintained productivity relative to PC at all ages. Apparently, BW and bone reserves in the NC2 hens were depleted beyond the level required to maintain their production performance, whereas BW and bone reserves of the NC1 hens were adequate to support egg production throughout the study. Diets with 2.0 g/kg avP maintained productivity and BW from 20 to 70 woa, whereas a diet with 1.5 g/ kg avP maintained productivity but decreased BW from 41 woa, and a 1.0 g/kg avP diet decreased productivity from 28 woa and BW from 31 woa (Boling et al., 2000). The mechanism by which NC1 maintained and NC2 decreased performance can be explained by the adverse effects of avP and Ca reductions in each of the diets on BW and bone quality of the hens. The moderate reductions in avP and Ca in the NC1 diet were not sufficient to reduce egg production or eggshell quality throughout the study. In actively laying hens, cortical and trabecular bone tissues are not formed but may be resorbed, whereas the medullary bone tissue can be formed and resorbed to support eggshell formation in response to daily fluctuations in Ca supply and demand because of patterns of feed intake and shell formation (Dacke et al., 1993; Kerschnitzki et al., 2014). Hence, new structural bone tissues would not have been formed in the NC1 hens but may have been resorbed to support eggshell formation, in addition to medullary bone. However, the NC1 diet also maintained cortical bone density but decreased BW from 76 woa and femur trabecular space BMD and BMC at the end of the study. Trabecular microarchitecture of the NC1 hens was maintained, and the medullary BMD and volume and thickness tended to decrease relative to the PC (Bello, 2018). The decreased trabecular space BMD and BMC caused by the NC1 treatment indicated decreased medullary bone mineralization in the NC1 hens. Relative to Caadequate diet, a diet with 32.6 g/kg Ca fed from 19 to 45 woa maintained egg production and decreased BW and increased osteoporosis incidence (Cransberg et al., 2001), indicating that the NC1 hens likely maintained egg production by increasing resorption of structural bone after 26 wk. Genetic selection over the years has increased the resistance of modern egg-laying hens to structural bone loss (Fleming et al., 2006; Raymond et al., 2018). Hence, the NC1 diet may need to be fed for more than 10 wk for adverse effects on structural bone tissues to be observed. The decreased BW from 76 woa and the nearly significant decrease in medullary bone at 78 woa imply an initial stage of the effect of the moderate reduction of avP and Ca in the NC1 on the performance of the hens.
The decreased egg production and eggshell quality but maintained structural bone tissues by the NC2 diet indicated that the severe avP and Ca deficiencies forced some of the hens to molt to restore structural bones in the hens. At least 4 out of the 20 hens were molting and stopped laying in the last 3 to 5 wk of the study. Molting in egg-laying hens decreases circulating estrogen level to stop egg production and medullary bone formation but restore structural bone (Dacke et al., 1993). However, Kim et al. (2007) reported that QCTmeasured trabecular bone density from both femur and tibia decreased after 9 d of molting. The femur and tibia metaphyses measured as “trabecular bone” in that study would have also contained medullary bone, which was not accounted for by the authors. Although medullary bone is mainly concentrated in the mid-diaphysis and trabecular bone toward the metaphyses, the 2 bone tissues are intermingled within the endosteal cavity (Shahnazari et al., 2006; Kerschnitzki et al., 2014; Bello, 2018). Because it is illogical for the trabecular bone to decrease and for medullary bone to increase during a molt, the decreased “trabecular” BMD with a 9- d molting from 86 woa hens reported by Kim et al. (2007) indicated that the deposition of structural bone was likely masked by the loss of medullary bone in the distal and proximal metaphyses of the long bones. The NC2 hens that had begun to molt in the current study would have had decreased medullary bone and have begun to replenish structural bone (Fleming et al., 1998).
Table 4. Effect of dietary Ca and available P levels and phytase on eggshell quality traits of single-comb White Leghorn hens from 68 to 78 wk of age.1,2
Table 5. Effect of dietary Ca, available P levels, and phytase on serum biochemical bone markers in single-comb White Leghorn hens at 78 wk of age.1,2
Overall, the effects of diet on bone densitometry of total, cortical bone, and trabecular space tissues measured using QCT at the proximal metaphysis at were similar to those at the mid-diaphysis (Table 6). As the QCT technology used in this study required separate scans at each location (Korver et al., 2004), there was no advantage in this study to measurements taken at multiple scan locations; this is similar to a previous long-term study in laying hens (Bello and Korver, 2019). Future work in this area could therefore be done using a single scan location.
Furthermore, the NC2 diet increased serum pyridinoline concentration, indicating a higher osteoclastic activity (Knott et al., 1995) with no indication of the degree of mobilization from each of the 3 bone tissues (Regmi et al., 2015). The serum analyzed for the bone biomarker analyses was collected between 7:00 am to 11:30 am, around when most hens lay (Kerschnitzki et al., 2014), and this may have influenced the lack of diet effect on serum osteocalcin concentration, the bone formation marker. Palpation of the eggshell gland of the hens before bleeding confirmed that the majority of hens were forming eggshell during the 4.5 h blood collection period. Hens that were forming eggshells at this time should have Ca supplied to the eggshell largely from dietary intake and not bone, and hens that were not forming eggshells at this time should have been forming medullary bone. The increase in serum pyridinoline concentration indicated that the severe reduction in Ca and avP caused the NC2 hens to mobilize bone Ca to support eggshell formation. Also, the lack of diet effects on serum PTH, P, and Ca may be because of the very tight regulation of their respective blood levels (Dacke et al., 1993). Hence, the reduced medullary bone at 78 woa in the NC2 hens relative to the PC hens as measured by QCT (Table 6) and microCT (Bello, 2018), together with the decreased BW after 73 woa, and the maintained structural bone through the 10 wk study implies a severe deficiency of at least one of avP and Ca in the NC2 hens. The overall effects of the deficiencies in this diet on bone mineralization may be partially masked by the inclusion of hens that had stopped laying and were going through a molt and were likely re-forming structural bone at the expense of medullary bone.
Table 6. Effect of dietary Ca, available P levels, and phytase on bone densitometry of total, trabecular space, and cortical bone tissues in femur proximal metaphysis and mid-diaphysis of single-comb White Leghorn hens at 78 wk of age.1,2
Effects of Phytase Supplementation in Diets Moderately and Severely Reduced in Available P and Ca
The NC1 diet allowed for maintained egg production, feed intake, FCR, and eggshell quality and therefore limited the opportunity to assess the efficacy of BSP on performance and eggshell quality. However, phytase supplementation in the NC1 diet alleviated the adverse effect of the moderate avP and Ca reductions in terms of decreased BW and total and trabecular space BMD and BMC of the hens. The inclusion of phytase in avPand Ca-reduced diets increases the availability of the minerals in the gastrointestinal tract of laying hens (Van der Klis et al., 1997; Liu et al., 2007; Beutler, 2009), resulting in maintained egg production, BW, and bone mineralization (Boling et al., 2000). Therefore, supplemental BSP increased P and Ca utilization to maintain medullary bone mineralization and healthy remodeling of the nonstructural bone to support eggshell formation. On the other hand, phytase inclusion in the NC2 diet alleviated the decreased performance, eggshell quality, BW, and bone quality caused by the severe avP and Ca reductions in laying hens. Supplemental BSP also decreased overall bone resorption as indicated by the decrease in serum pyridinoline concentration. However, supplemental phytase did not completely prevent the decreased total and trabecular BMD and BMC relative to the PC. Evidently, the reductions of avP and Ca in the NC2 relative to the PC were beyond the phosphoric potential of the 600 FTU/kg of BSP. This finding is consistent with that of (Bello, 2018), which also showed that the use of the BSP in the NC2 diet did not completely alleviate the detrimental effects on microarchitectural traits of femur medullary bone relative to the PC. Although 600 FTU/kg BSP prevented the adverse effects of severe avP and Ca reductions on the performance of laying hens, this level of supplementation was insufficient to maintain medullary bone mineralization. This finding implies the need to maintain a balance between the degree of avP and Ca reduction and dietary phytase activity to maintain hen performance and prevent bone loss.
Egg-laying hens are able to maintain productivity on avP and Ca-reduced diets, depending on the degree of nutrient deficiency. (Boling et al., 2000; Silversides et al., 2006; Geraldo et al., 2014). Egg-laying hens were able to physiologically adapt to the Ca and avP reduction and maintain performance at the expense of BW and bone mineralization, until their BW and bone reserve could no longer support performance and eggshell formation. The NC1 hens fed the moderately avP- and Ca-reduced diet maintained performance and eggshell quality throughout the study, with no evidence of structural bone loss. Although the severe reduction of avP and Ca in the NC2 diet decreased hen performance, it did not result in structural bone loss. However, the NC2 hens that went out of lay may have partially replaced lost structural bone as the molt proceeded. On the other hand, the NC2 hens that were actively laying when the trial was stopped would likely have still been undergoing structural bone loss. Hence, phytase supplementation in diets reduced in avP and Ca supported medullary bone remodeling in actively laying hens. Phytase supplementation allows for a substantial reduction in dietary avP and Ca levels with no adverse effect on bone metabolism of laying hens when the enzyme is adequate to release sufficient mineral to counteract the dietary reductions. Medullary bone is essential in the laying hen as a labile mineral reserve for eggshell formation (Dacke et al., 1993) but provides minimal resistance to bone fracture (Fleming et al., 1998). Phytase in the diet of laying hens may allow for further reductions in avP and Ca levels beyond those in the NC1 diet to decrease feed costs relative to the current practice, with no adverse effects on performance and bone health of the birds.
ACKNOWLEDGMENTS
The authors are grateful to Kerry Nadeau and the research team in the Korver lab, and the barn staff of the Poultry Research Centre, University of Alberta, Edmonton, AB, Canada. Also, the authors acknowledge Danisco Animal Nutrition, DuPont Industrial BioScience, Marlborough, UK, for providing funding of this study.
Conflicts of Interest Statement: The authors did not provide any conflict of interest statement.














.jpg&w=3840&q=75)