Trypsin Inhibitor, the hidden enemy in Soyabean Meal
Corn gluten meal with synthetic amino acids valine and possibly isoleucine adjusted the diet.


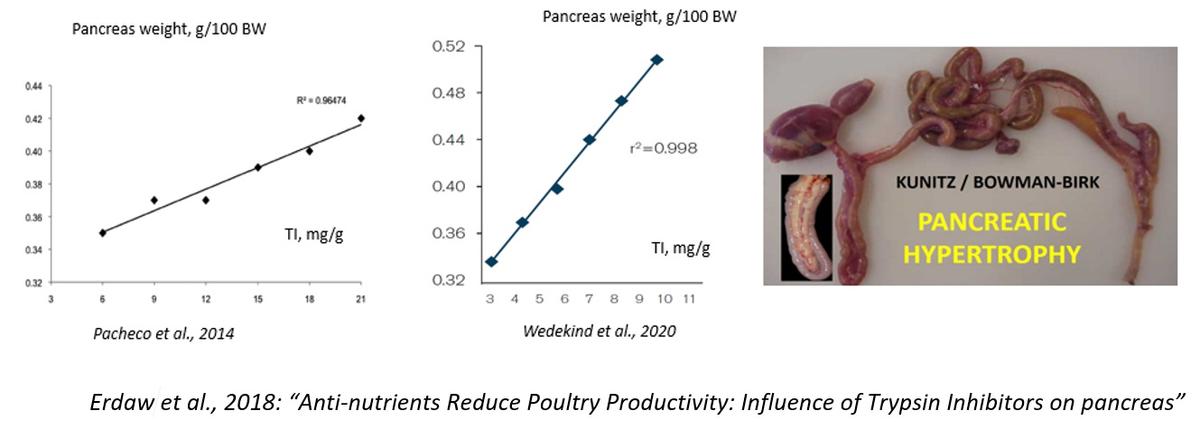
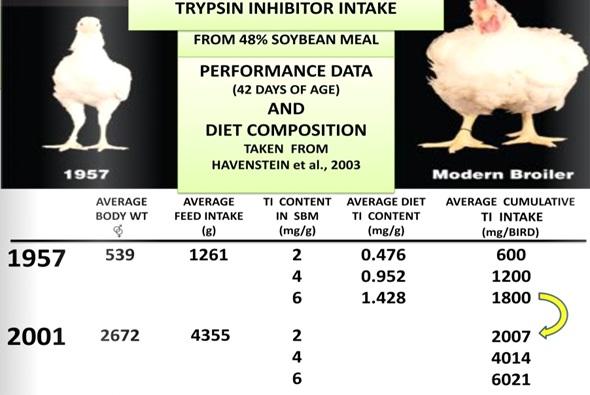
Dear Dr. Koushik De. I find interesting your discussion on trypsin inhibitors and their role as antinutritional factors in broiler nutrition. It would be nice however, if you add a list of all the literature cited and provide credit where credit is due. Havenstein et al. (2003) didn't discuss trypsin inhibitors in their comparison of the 1957 and 2001 genetics, the slide that you have in your paper comes from my discussion in 2012 at the Arkansas Nutrition Conference [Ruiz, N., 2012. New insights on the urease activity range for soybean meal: a worldwide opportunity for the poultry industry. 2012 Arkansas Nutrition Conference Proceedings]. In my opinion, and of course, it is only my opinion, trypsin inhibitors are not "the hidden enemy" in soybean meal. Since the 1940's they started to be clearly identified by Kunitz (1945), Bowman (1944), and Birk (1961). And between 1969 and 1974 Kakade et al. developed the necessary analytical chemistry for their quantification. A considerable amount of the work on trypsin inhibitors has been done in soybeans and their products such as soybean meal.
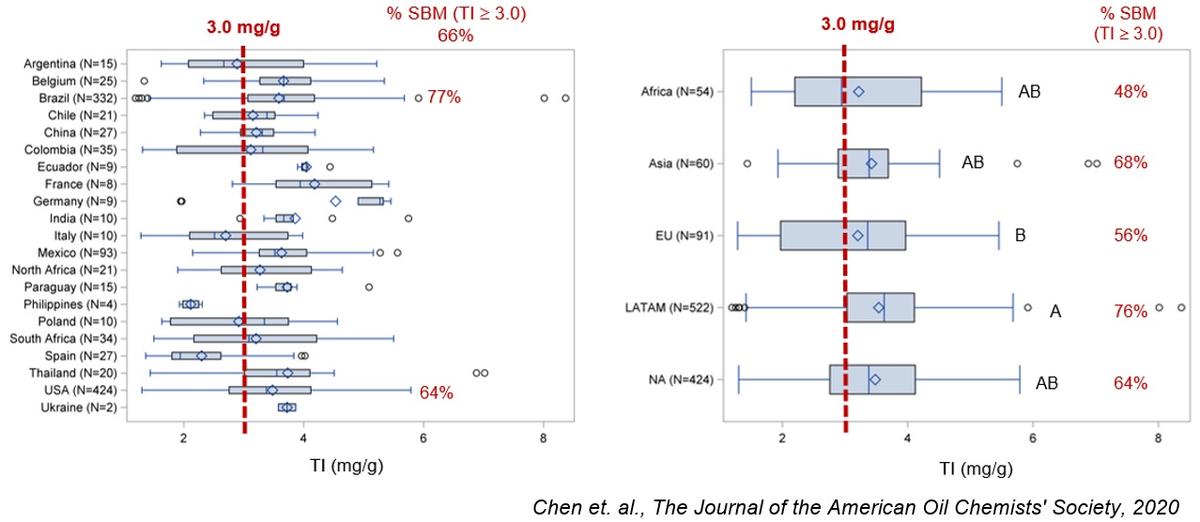
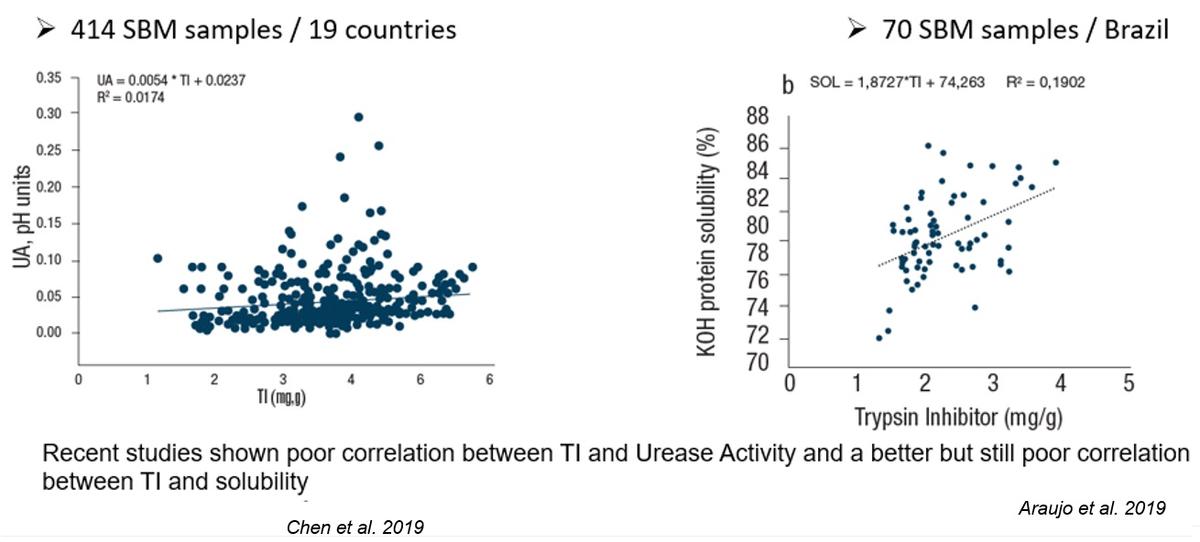
I would say that rather than hidden, trypsin inhibitors in soy have been ignored for a variety of reasons. One important reason why during decades the industry didn't refer directly to trypsin inhibitors in soybean meal was the high correlation between urease activity and the inhibitors although it was not explicitly recognized. For decades the range of adequacy measured with urease activity was 0.05 to 0.20 pH units, and millions of tons of excellent quality SBM was produced and utilized in animal nutrition worldwide including broilers. This was true until the 1990's when the rapid feed passage syndrome showed up in broiler farms, particularly out of the United States [Kouwenhoven, B., R.M. Dwars, J.F.M. Smeets. 1992. Wet litter and high feed conversion, a new problem in broilers. Pages 558-561. Vol. 1. In: Proceedings XIX World’s Poultry Congress. WPSA. Amsterdam.] The rapid feed passage syndrome was the result of the dramatic increase in feed intake of broilers which accompanied with the predominance of soybean meal worldwide as the number one source of digestible amino acids exceeded the tolerance of broilers to trypsin inhibitors in the diet.
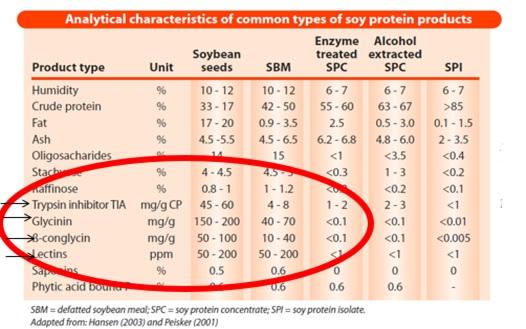
In 2005 [Ruiz, N., and F. de Belalcázar. 2005. Field observation: Trypsin inhibitors in soybean meal are correlated with outbreaks of feed passage in broilers. Poultry Science 84(Suppl. 1): 70.] we presented field data that indicated that commercial soybean meal displaying urease activity values within the range of adequacy were clearly involved in rapid feed passage in broilers older than 21 days of age. But in 2005 we measured simultaneously trypsin inhibitors and urease activity in lots of soybean meal that resulted in excellent performance of broilers as well as in lots that generated rapid feed passage. It became clear that the old range of adequacy had changed, and by 2012 at the Arkansas Nutrition Conference we proposed a new range: 0.000-0.050 pH units. Using the ISO 14902:2001 method to determine trypsin inhibitors we correlated this new proposed range for urease with a 1.65 - 2.35 mg/g range of adequacy for trypsin inhibitors. Since 2005 we also proposed the importance of measuring trypsin inhibitors as QC parameter in soybean meal and full-fat soybeans.
Yes, trypsin inhibitors is a QC parameter for both soybean meal and full-fat soybeans. Solubility in KOH is the parameter to measure if overprocessing has occurred in SOYBEAN MEAL and it is highly correlated to amino acid digestibility in soybean meal. We have reported that KOH protein solubility is not a QC parameter for full-fat soybeans [Ruiz, N., and C.M. Parsons. 2016. The absence of correlation between protein solubility and digestible amino acids in full-fat soybeans emphasizes focusing on trypsin inhibitors reduction. International Poultry Scientific Forum, Abstract T195 (p. 58); Poult. Sci. 95(E-Suppl. 1):251.]. Soybean meal and full-fat soybeans are two different ingredients each with distinctly quality control parameters [80th Minnesota Nutrition Conference, p. 169-175, 2019].
As far as how to deal with trypsin inhibitors in the feed formulation of broilers there are several alternatives beyond proteases. Nutritionists and formulators under commercial conditions have to think in overall cost, and formulating to minimize trypsin inhibitors in a broiler finisher diet is expensive because limiting soybean meal, the economic number one provider of digestible amino acids as already said, would result in higher price per metric ton of feed. As suggested by Dr. Marchenkov there are alternative ingredients such as sunflower meal that compete against soybean meal when a maximum spec for trypsin inhibitors in the formula is in play. Canola is another one. Per our experience, 0.58-0.60 mg of TI/g of FEED in a broiler finisher feed is roughly the upper limit.
Another way to effectively control trypsin inhibitors in broiler feeds is the utilization of full-fat soybeans (FFSB) which occur in several countries out of the United States. Given the fact that FFSB in practice is a no-commodity because is processed by the end user, then it is possible to decrease trypsin inhibitors in FFSB well below the trypsin inhibitor content of commercial soybean meal (a commodity). Soybean meal cannot reach by the solvent extraction process values below 1.60-1.65 mg TI/g without being overprocessed, while FFSB, PROPERLY PROCESSED may reach values as low as 1.0 mg TI/g (measured with the ISO 14902:2001 method). Consequently, by generating FFSB of superior quality than commercial soybean meal as far as trypsin inhibitors are concerned the only limitations are cost and inventory. In our experience FFSB competes excellently with soybean meal, reduces cost per MT of feed and allows to control trypsin inhibitors below the 0.58-0.60 mg TI/g of FEED. Nelson Ruiz Nutrition, LLC, Suwanee, GA USA.

https://www.sciencedirect.com/science/article/pii/S0377840122002085
Very nice and educative topic, I learned a lot.
Thank you to all the contributors.