Introduction
Reproductive efficiency of roosters and the hens they mate determines the breeding flock fertility which is as important to the entrepreneur as the egg lay is (Akhlaghi et al., 2014). Fertility is essential to produce the maximum number of quality chicks per hen housed. Without producing fertile eggs, the best incubators and hatchery management procedures cannot produce chicks (Bramwell et al., 1996). Through intense genetic selection and improved nutritional management, there has been a steady increase in the growth rate of broiler breeders and their progeny. Intensive selection based on growth traits in meat-type breeder flocks has resulted in their moderate but constant decline in fertilizing potential. A negative correlation between reproductive and growth traits may be responsible for such the decline in the fertilizing potential of birds selected for rapid growth (Brillard, 2004).
The consequent belief of the poultry men in artificial insemination to find its place in the broiler industry as one of the tool for fertility maintenance at acceptable fertility levels is gradually turning to reality, much like it has been in turkey breeding programs. Apart from hen’s contribution to egg production and provision of optimal microenvironment in the oviductal sperm storage, for the subsistence of residing spermatozoa (Bramwell et al., 1996), the rooster role is also critical in flock fertility (Saemi et al., 2012). Subfertility is thought to be largely a problem in males because the ratio of males to females in a flock is very low (Lin et al., 2005). The deterioration in fertilizing ability has been attributed to many factors like age, weight and decline in semen quality (Khan, 2011).
A better approach (Wishart and Staines, 1999) to quantify breeding efficiency is to estimate the numbers of sperm that interact with the egg in the infundibulum. These can be identified in laid eggs, as sperm in the outer perivitelline layer (OPVL sperm), or holes produced by sperm in the inner perivitelline layer (IPVL holes). Eggs can contain up to 250,000 OPVL sperm, so the scale improves on binary estimation of fertilization status.
This in vivo sperm penetration assay is a relatively new technique that is used to determine the number of sperm that bind, acrosome react, and penetrate the outer layer of the ovum prior to fertilization. A linear relationship (r2 = 0.81) exists between parameters of sperm quality and IPVL holes from eggs laid by inseminated hens (Robertson et al., 1998). Thus, enumeration of IPVL holes provided a clearer validation of sperm quality tests as a basis for selection of breeding males. The quantitative advantage of using sperm penetration measurements for differentiating between fertility of semen samples from different males, from groups of males subjected to different dietary or environmental regimens, or after liquid or frozen storage, seems convincing (Wishart and Staines, 1999). The sperm penetration assay was developed in 1990 at the University of Georgia primarily as a research tool and has been used with great success. Since that time, the assay has been modified as a research tool to include its use as an in vivo assay using laid eggs. However, possibly more important is its use to evaluate commercial flocks to predict their future fertility.
The technique excludes the female variation in the form of hen’s mating efficiency, sperm storage, sperm transport and sperm binding etc, assesses rooster reproductive efficiency in the post insemination phase and was thus opted for the current trails. The aim of the present study was to compare the effect of natural mating and artificial insemination on the reproductive efficiency of male broiler breeders.
Materials and methods
Study location
The field study was carried out in commercial poultry farm located in Lahore, Pakistan. The city is characterized by a long (December-January) and short (August) raining season. The experiment was conducted from November 2015 through April, 2106 under controlled environment where the ambient temperature averaged between 40 and 45° C with Relative Humidity (RH) ranged from 65-90% in hot humid July to August months. The average house temperature and RH during the experimental trials were 21-24o C and 50-65% respectively.
Birds and experimental conditions
Hubbard Boiler Breeder cockerels (n=1,800) were first brooded and raised on littered floor till they aged 18 weeks and were then randomly divided into three groups. Group-1 (n=600) was housed in cages (n=600) to artificially inseminate cage housed flock, Group-2 was separately housed to inseminate the floored hens and Group-3 (n=600) mated the floored hens naturally. Each of the roosters’ group had to serve 10,000 hens. Insemination on floor and cages continued from 23rd through 65th week of flock age comprising roosters and hens of the same age. The flocks were inseminated at 7 th day.
Each of the four sheds had a floor space of 18800 square feet. In battery cages manufactured by Guangzhou Guangxing Poultry Equipment Company Limited (http://www.cnguangxing.com), hens were housed in Hot Dip Galvanized 3 tier cages, measuring, 658 cm2 areas per female bird and 3 birds per cage, 1645 cm2 area per male bird and one male per cage.
The floor houses were equipped with semi-automated feeders being picked up 15 minutes after feeding thereby reducing hen house floor space requirement to 1.88 square feet per bird. It is 13% less than the usually required for layers in tropical climates (Maba, 2008 and Banga-Mboko et al., 2007). The cage housed hens occupied 0.89 square feet floor area per hen.
The floored flocks were provided with nests. Wheat straw served the purpose of substrate in the floored pens. Both the cage housed, and floored flocks were watered through automatic drinkers and fed manually.
The cage housing had the facility of automatic manure removal daily. Both type of flocks observed similar lighting schedule of 16 light hours with 60 LUX and 8 dark hours with zero light intensity during the entire production period.
Feeding and vaccination
Feed for hens and roosters was formulated as per management guidelines for Hubbard Classic breed that contained 2750 Kcal kg-1, 16% crude proteins, 3.5% calcium (Ca) and 0.06% methionine (M) as per formulation by Banga-Mboko et al. (2007.
The floor housed hens and cockerels were daily offered 118 and 110 g respectively on 25th week that peaked at 175 for hens on 29th week and at 142 per day/bird for cockerels on 52nd week.
The caged house hens and cockerels were daily offered 105 and 97 g a bird on 25th week that peaked at157 on 29th week for hens and at 100 on 54th week for cockerels. The flocks were vaccinated against Newcastle, IB, Coccidiosis, Mareks, IB Variant, IBD (Live & Attenuated), ILT, AI H9, E. coli, IBH and EDS.
Semen Collection and insemination
Floored roosters and cage housed roosters served their respective floored and cage housed hens. Cage housed hens were inseminated artificially on each 7th day throughout the flock production period.
The schedules followed matched the five-day industrial work-week (Van Krey and Siegel, 1976). Semen was collected from cockerels on alternate day. Semen taken from each 3-4 cockerels used to be pooled together, mixed with 0.4 cc diluent (30% Beltsville Poultry Semen Extender; Hudson et al., 2016) that approximately made final volume of 2cc and gently stirred to inseminate 28-32 hens. Each injector contained 2 million sperms with average penetration value of >60
Semen was collected and inseminated using the abdominal massage procedure described by Burrows & Quinn (1937) and adopted by Yahaya et al. (2013).
The testes located at the dorsum were stroked and massaged until there was protrusion of the cloacae. The semen was then milked and collected directly using a glass funnel.
Three persons were involved in the insemination process. The first person carefully caught and restrained one hen at a time with his hands.
The second person carried out abdominal massage of the breeder hens according to the method of Lake and Stewart (1978) followed by Yahaya et al. (2013) and as soon as there was partial eversion of the cloaca, he exerted a controlled pressure on the lower abdomen for eversion of the vagina. Thereafter, the tuberculin syringe with pooled semen was inserted into the hen’s vagina by the third person, who released the semen intravaginally as soon as the vagina started to relax.
Measurements
Five variables; feed intake, body weight, semen quality, sperm penetration rate and hatchability were recorded for a complete production phase from 25th to 65th week.
Semen evaluation
Fresh semen collections were evaluated to determine semen characteristics. Seminal volume was measured in graduated collecting tubes. Sperm forward motility was assessed by placing a portion of ejaculate diluted with 2.9% sodium citrate solution (1:100) on a slide, using a Zeiss (Jena, Germany) compound light microscope (400 × magnification), equipped with a warm stage (37°C). Sperm viability and abnormality were determined, using a portion of ejaculate stained with eosin- nigrosin solution, by observing100 spermatozoa per slide. Spermatozoa with detached heads, abaxial heads, malformed heads, bent tails, coiled tails, double tails, and protoplasmic droplets were considered as abnormal (Pursel et al., 1972). Sperm concentration was determined using a Neubauer hemocytometer.
Perivitelline layer (PVL) sperm penetration assay Determination of PVL sperm penetration assay was carried through the slightly modified version (Namdari et al., 2016) of the procedure developed by Bramwell et al. (1995).
The egg was broken, and the albumen was separated from the egg yolk which was then put on a glass container with the germinal disc placed on the top.
The surplus albumen was separated from the PVL through tissue paper.
The germinal disc (Blastodermic area) was cut with scissors and immediately rinsed in 1%NaCl solution to detach excess yolk from the egg membrane. Then the PVL was placed on a microscopic slide followed by the addition of some egg albumin, fixed by adding 3-4 drops of 10% formalin for 1 hour, and rinsed with 1% NaCl solution.
The fixed PVL was then replaced with periodic acid-Schiff and allowed to dry at room temperature. The inner PVL holes were counted using a light microscope (OSK856861, Japan) at 400 ×.
The blastodermic area was positioned on each slide and centered in the field of vision (area = 40 mm2) and the number of holes within this area counted.
Hatchability
Eggs were set in forced-air incubator for 18 days at 99-99.5°F and 60-65% relative humidity (83-88°F wet bulb) and transferred to hatcher where temp and humidity were 98.2°F to 98.5° F and 90% respectively.
Statistical analysis
Data were processed using version 22 of the SPSS software. ANOVA was performed and mean values for input and output variable obtained from the cage housed and floored flocks were compared among treatments using the LSD at 5% significance level. Regression analysis was performed with the help of Pearson’s correlation and multiple regression analysis.
Results
Reproductive parameters
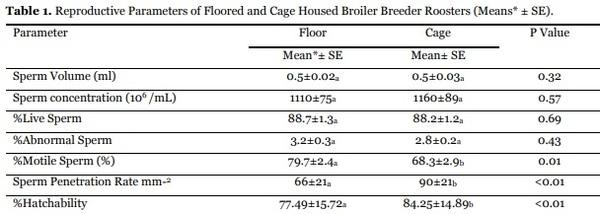
Housing type did not affect (P>0.05) seminal volume, sperm concentration, viability and morphology. However, variation (Table 5.1) was significant in sperm penetration and hatch (P<0.01).
Effect of Rooster Housing on Sperm Penetration and Consequent Hatch
Average sperm penetration for floored and cage housed cockerels is given in Table 1. Rooster housing affected sperm penetration (P<0.01) and consequent
Postinsemination Fertility
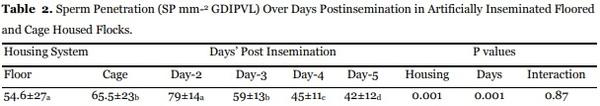
Table 2 presents the sperm penetration over days post insemination. Sound number of sperms (42-87) penetrated the perivitelline membrane overlying the germinal, to ensure optimum fertility level. Sperm penetration was the highest (P<0.01) in the eggs laid the next day after insemination in both cage housed or floored flocks. Fewer (P<0.01) sperms penetrated the perivitelline membrane overlying the germinal disc with lengthening post insemination duration over 3, 4 and 5 days. Sperm penetration till next insemination was sound enough (>42) to cause fertility decline in either flock.
Age Effect on Sperm Penetration
Sperm penetration was negatively (P<0.05) associated with rooster age (Table 4). Weekly age increment reduced one sperm to penetrate the perivitelline layer overlying the germinal disc and the regression equation predicts no penetrating sperm at 92nd week onwards.
Body Weight Effect on Sperm Penetration
Sperm penetration decreased with increasing body weight of rooster (Table 3). Semen from roosters weighing 4-4.9 kg exhibited highest (91±11) sperm penetration followed by roosters having 4.5-5 kg (60±22) and above 5 kg (42±19).
Sperm penetration was negative (P<0.05) when regressed on rooster body weight (Table 4). Each 100 grams body weight gain by cockerel reduced 3 SP mm-2 GDIPVL.
It is thus predicted that the current rooster will be totally unfit for floor mated reproduction at a heavy body weight (>6.3 kg) because they will supply no sperm that succeed to penetrate the perivitelline layer overlying the germinal disc.
Body weight related decrease in sperm penetration did not harm rooster reproductive performance in artificially inseminated flocks for all the age groups (Table 3) but dropped too low (<27) to cause subfertility in naturally mated flock after the rooster weighed above 5 kg.
Discussion
In the present study, sperm quality parameters such as semen volume, sperm concentration, live sperm, abnormal sperm, motile sperm, sperm penetration rate and hatchability did not differ in the two housing system. In line with the values reported for broiler breeder semen evaluated by Namdari et al., 2016 variation in sperm penetration and hatch were significant but seminal volume, sperm concentration and viability remained unchanged. However, sperm morphology did not match with reference trials due to type of treatment. In the present study, the numerically better sperm penetration and consequent hatch for cage housed cockerels is attributed to insemination type applied in the two housing systems whereby artificial insemination ensured optimal supply of semen to the fertilization site. Older roosters performed better than younger (Bramwell et al., 1996) with respect to sperm penetration and fertility when flock is inseminated artificially Semen extender was used in inseminating the cage housed roosters’ semen that might have favored the transit of sperms through the oviduct in their attempt to reach the infundibulum area, limiting their access to the ovum perivitelline layer (Abouelezz et al., 2015).

There have been conflicting reports of the relationship of concentration and motility to fertility. The majority of reports have found no relationship between the two (Cooper and Rowell, 1957), while other researchers have seen positive correlations (Boone and Huston, 1963). Sperm motility has been correlated to the fertilizing ability of males (McDaniel et al., 1997) and motility based roosters’ selection at early age improves flock fertility (Parker et al., 2000).
In the current study, sperm penetration was better (P<0.01) was better in cage housed than floored flocks. Sperm-egg binding has exhibited a high correlation (r= 0.83) to fertility (Barbato et al., 1998). The better sperm penetration rate by roosters housed in the modern cage systems may be attributed to several management factors that favored reproductive performance. Besides increased spatial density of birds, an easier control of microclimate, a simplified waste disposal and an easier supervision of individual birds for production level and health status reported by Azeroul (2005) and Pistikova et al. (2006), cage housing enhanced roosters’ reproductive efficiency through provision of clean environment and uniform feed allowance per bird. Fertility and hatchability are most sensitive to environmental and genetic influences (Stromberg, 1975).
Heritability estimates for fertility and hatchability in chickens range from 0.06-0.13 (Sapp et al., 2004), this indicates that the non-genetic factors have a higher influence on these traits. Both are interrelated heritable traits that vary among breed, variety and individuals in a breed or variety. System of husbandry and rearing technology (Weis, 1991) are among other factors including egg age (Tarongoy et al., 1990), storage condition (Brah and Sandhu, 1989), flock age (Rogue and Soares, 1994), mating system (Gebhardt-Henrich and Mark, 1991) that influence the hatchability. Variation in reproductive efficiency may be attributed to differences in management practice and environment (Kalita 1984). Considering the overall hatchability traits: breed has little effect on hatchability in poultry (Islam et al., 2002).
When a hen is inseminated, she will lay fertilized eggs for a period of time without need for further artificial insemination or copulation (Lake, 1975). In the current trails, egg fertility in artificially inseminated flocks declined over days post insemination till next insemination mainly with decreasing number of sperms in the sperm storage tubules in the hen. However, it did not change in the naturally mated flock due to ample daily supply of sperms. The length of the fertile period is positively correlated with the OPVL sperm in the first fertile egg (Wishart, 1987; Brillard and Antoine, 1990) and in cases of severe subfertility, the problem appears to be one of differing sperm transport among hens, rather than insufficient sperms/AI. Gumulka and Kapkowska 2005 lengthened fertility duration by 2 days post insemination and concluded that high values for effective fertility can be obtained from broiler breeders inadequate environmental and technological conditions of AI. Subfertility in the floor mated flocks is a problem of sperm allocation among hens rather than total number of sperm transferred by the roosters. The striking feature of the distribution of IPVL holes in eggs from broiler flocks was the enormous variation. Although majority of eggs contained sufficient sperm to ensure fertilization but considerable number of eggs from the same flock were unfertilized.
The standard deviation for number of OPVL sperm in eggs from naturally mated broilers was twice that with AI (Wishart et al., 1992). Thus, it seems that the main cause of subfertility in floor-mated broiler flocks is that many hens rarely, if ever, mate. In floor-mated broiler flocks, dose and quality of sperm deposited are variable and, as with AI, sperm transfer and a hen’s oviducal storage capacity affect outcome.
In the current study, roosters were less reproductively efficient with increasing body weight. Heavy body weight lowers mating frequency that may have declined sperm penetration. Rooster’ body weight is ineffective on sperm penetration (Bramwell et al., 1996) when flock is artificially inseminated and rather older males had high sperm penetration and fertility. Heavy breed flocks and the extensive systems require more cocks (Islam et al., 2002).
Higher fertility has been recorded for light (White Leghorn) when compared with heavy breeds (Barred Plymouth Rock: Rhode Island Red, White Rock and New Hampshire) (Islam et al., 2002). Both increased body weight and age reduce fertility in broiler breeders (Bramwell et al., 1996). However, Swan (1977) reported higher hatchability percentage with meat strains as compared to egg strains which is in disagreement with the current findings and aforementioned studies.
Conclusion
Reproductive efficiency of roosters and the hens they mate determines the breeding flock fertility that varies with housing system, mating type, age and body weight. Better housing, careful application of AI and regular monitoring of flock reproductive efficiency for in time corrective measures ensures smooth profitable business operation. Adoption of sperm penetration based reproduction monitoring plan guides us for minor management changes that can avoid a costly reduction in the number of quality chicks hatched.
This article was originally published in International Journal of Biosciences. http://www.innspub.net. Vol. 12, No. 2, p. 26-34, 2018. http://dx.doi.org/10.12692/ijb/12.2.26-34.