Physical Form of Diet Influence the Liver Function, Blood Biochemistry, and External Body Measurements in Broiler Chickens Exposed to Carbon Tetrachloride Toxicity
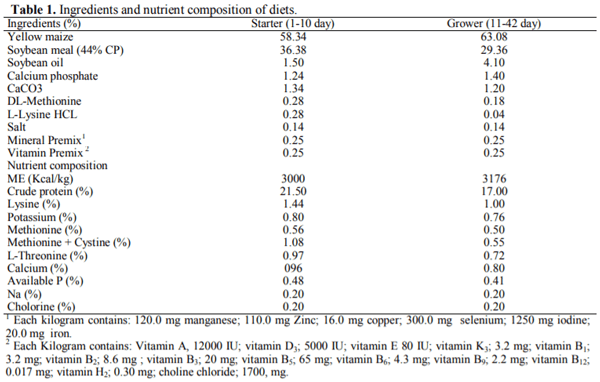
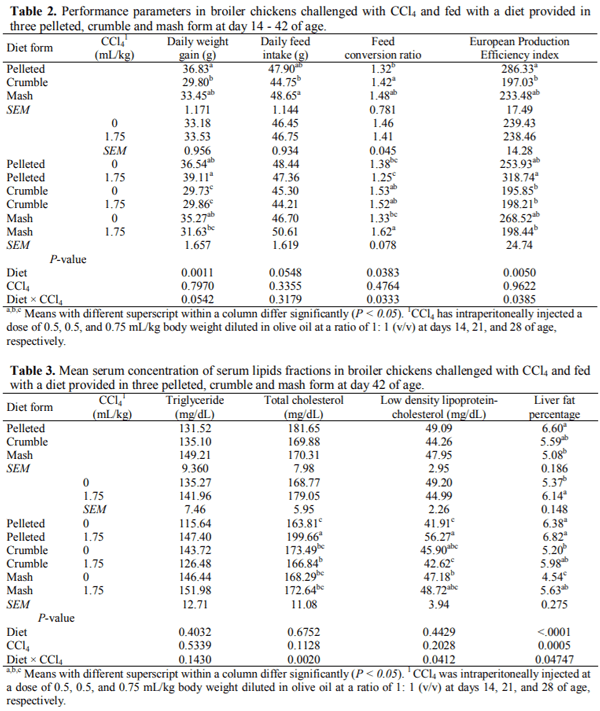
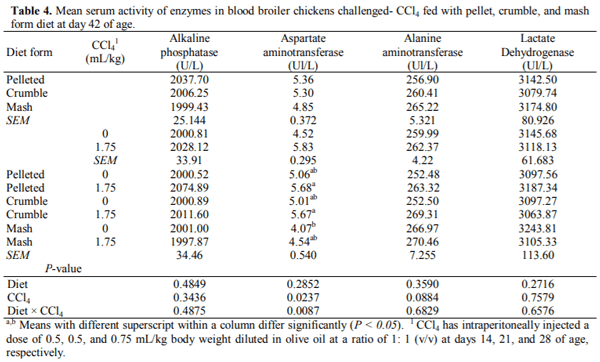
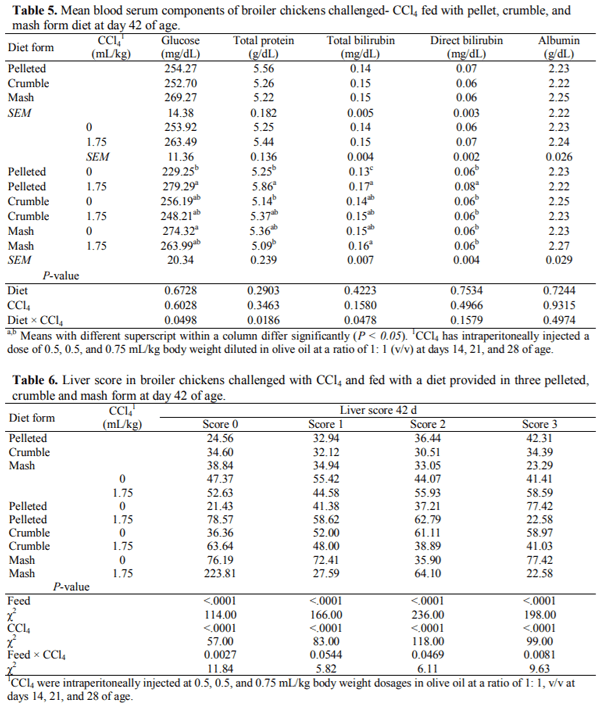
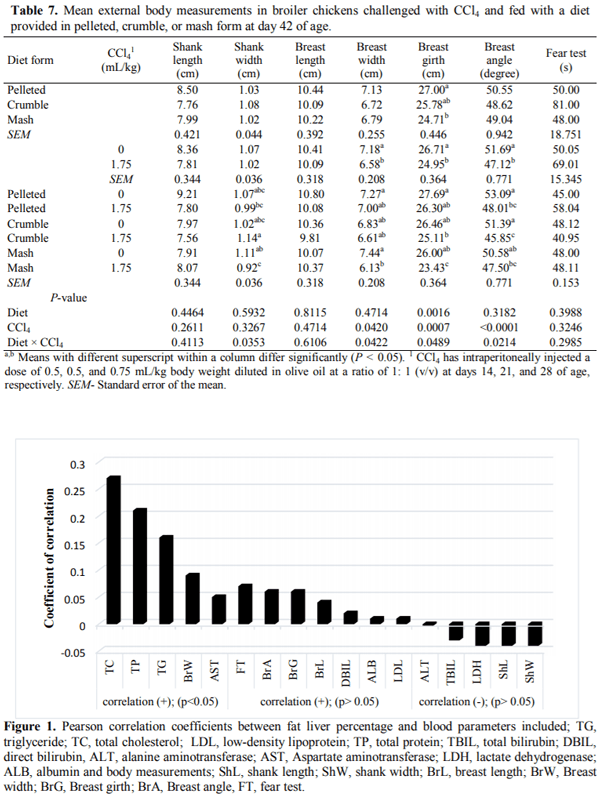
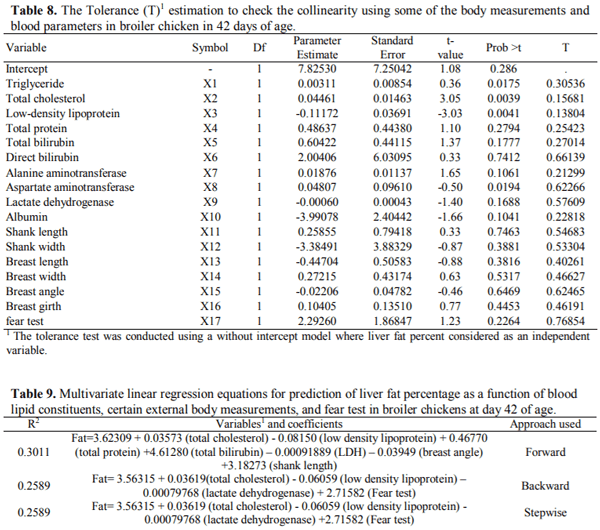
This 2 × 3 factorial experiment aimed to evaluate the single and interactive effects of feed form (pelleted, crumble and mash) and carbon tetrachloride (CCl4) intoxication on liver function, blood parameters, and certain external body dimensions in broiler chicken up to day 42 of age. The six experimental treatments were examined with a completely randomized block design in six replicates of 13 birds each using 468 10-day old female Arbor Acres (320±10g) chicks. For CCl4 intoxication, birds were intraperitoneally injected with 0.5, 0.5, and 0.75 mL/kg body weight CCl4 in olive oil at a ratio of 1: 1, v/v in days 14, 21, and 28 of age. Results indicated that feeding the pelleted diet increased daily weight gain (DWG), liver fat percentage (LFP), and improved feed conversion ratio compared with those received crumble and mash diets (P < 0.05). The broilers receiving the pelleted diet had a greater breast angle (BrG) than those feeding with the mash diet (P < 0.05). Exposure to CCl4 decreased breast width (BrW), breast girth (BrG), and BrA of the treated birds (P < 0.01). The best multivariate linear model for prediction of liver fat percentage achieved by the Forward modeling approach in SAS involving serum TC, LDL TP, TBIL, LDH, BrA, and ShL with R2=0.3011. It was concluded that feeding diets in pelleted form may cause a greater performance loss and liver dysfunction when broiler chickens are fed with contaminated feed resources. Moreover, prediction of LFP using multivariate linear models based on blood constituents and external body measurements could not be convincing as for no model R2 exceeds 0.31 likely due to the lack of strong correlation between LFP and the considered predictors.
Keywords Fatty liver Broiler chicken Feed physical form Liver fat prediction Carbon tetrachloride.
Abdollahi MR, Zaefarian F & Ravindran V. 2018. Feed intake response of broilers: impact of feed processing. Animal Feed Science and Technology, 237: 154–165. DOI: 10.1016/j.anifeedsci.2018.01.013
Abdollahi MR, Zaefarian F & Ravindra V. 2019. Maximising the benefits of pelleting diets for modern broilers. Animal Production Science, 59: 2023–2028. DOI: 10.1071/AN19254
Abe H, Nagao K, Nakamura A & Inoue-Murayama. M. 2013. Differences in responses to repeated fearrelevant stimuli between nagoya and white leghorn chicks. Behavioral Processes, 99: 95-99. DOI: 10.1016/j.beproc.2013.07.004
Baradaran A, Samadi F, Ramezanpour SS & Yousefdoust S, 2019. Hepatoprotective effects of silymarin on CCl4-induced hepatic damage in broiler chickens model. Toxicological Reports, 6: 788-794. DOI: 10.1016/j.toxrep.2019.07.011
Bedogni G, Nobili V & Tiribelli C. 2014. Epidemiology of fatty liver: An update. World Journal of Gastroenterology, 20: 9050–9054. DOI: 10.3748/wjg.v20.i27.9050
Behboodi HR, Samadi F, Shams Shargh M, Gangi F & Samadi S. 2017. Effects of silymarin on growth performance, internal organs and some blood parameters in Japanese quail subjected to oxidative stress induced by carbon tetrachloride. Poultry Science Journal, 5: 31-40. DOI. 10.22069/PSJ.2017.11578.1201
Beheshti Moghaddam S, Kermanshahi H, Vahed R, & Nasiri Moghaddam H. 2016. The protective effects of marigold (calendula officinalis) extract in liver damage by CCl4 in broiler chicken. Veterinary Researches Biological Products, 4 (109): 60- 69.
Boll M, Weber LWD, Becker E & Stampfl A. 2001. Pathogenesis of Carbon Tetrachloride-Induced Hepatocyte Injury Bioactivation of CC14 by Cytochrome P450 and Effects on Lipid Homeostasis. Zeitschrift für Naturforschung C, 56: 111-121. DOI: 10.1515/znc-2001-1-218
Chewning CG, Stark CR & Brake J. 2012. Effects of particle size and feed form on broiler performance. Journal of Applied Poultry Research, 21: 830-837. DOI: 10.3382/japr.2012-00553
Draper NR & Smith H. 1981. Applied regression analysis. 2nd Edition John Wiley & Sons, Inc.; New York. 709 Pages.
Elliott RJ. 1984. Ektachem DT-60 Analyzer. Physician’s Leading Comput. 2: 6.
Emmans GC. 1981. A model of the growth and feed intake of ad libitum fed animals, particularly poultry. In: Computers in Animal Production. Occasional Publication, 5: 103-110.
Euribrid BV. 1994. Technical information for Hybro broilers. Euribrid Poult Breeding Farm, Boxmeer. 22.
Folch J, Lees MG & Stanley GS. 1956. A simple method for the isolation and purification of total lipids from animal tissues. Journal of Biological Chemistry, 226: 497-509.
Gad AS, Khadrawy YA, El-Nekeety AA, Mohamed SR, Hassan NS & Abdel-Wahhab MA. 2011. Antioxidant activity and hepatoprotective effects of whey protein and Spirulina in rats. Nutrition, 27: 582-589. DOI: 10.1016/j.nut.2010.04.002
Gerzilov V & Petrov P. 2015. Relationship between some blood biochemical parameters and fatty liver weight in force feeding of mule ducks. Bulgarian Journal of Agricultural Science, 21: 1039-1043.
Ghareeb K, Awad WA, Sid-Ahmed OE & Böhm J. 2014. Insights on the Host Stress, Fear and Growth Responses to the Deoxynivalenol Feed Contaminant in Broiler Chickens. PLoS ONE. 9, e87727. DOI: 10.1371/journal.pone.0087727
Gous M. 2007. Predicting nutrient responses in poultry: future challenges. Animals, 1: 57–65. DOI: 10.1017/S1751731107657784
Hair JFIR, Anderson RE, Tatham RL & Black WC. 1995. Multivariate Data Analysis (3rd ed). New York: Macmillan.
Hong SA., Jung I., Choi S, Hwang Y, Lee S, Son, Y, Heo, Y, Cui, R, Han, S, Kim, H, Lee K & Kang Y. 2019. Sodium fluorocitrate having inhibitory effect on fatty acid uptake ameliorates high fat dietinduced non-alcoholic fatty liver disease in C57BL/6J mice. Science Reports, 9: 17839. DOI: 10.1038/s41598-019-54476-5
Jiang X, Chang H & Zhou Y. 2015. Expression, purification and preliminary crystallographic studies of human glutamate oxaloacetate transaminase 1 (GOT1). Protein Expression and Purification, 113: 102-6. DOI: 10.1016/j.pep.2015.05.010
Khodadust MR, Samadi F, Ganji F, Jafari Ahangari Y & Asad GH. 2015. Effects of peppermint (Mentha piperita L.) alcoholic extract on carbon tetrachloride-induced hepatotoxicity in broiler chickens under heat stress condition. Poultry Science Journal, 3: 1-16. DOI: 10.22069/PSJ.2015.2323
Khorramshahi M, Samadi F & Ganji F. 2014. The effects of Cynara scolymus L. on carbon tetracholoride induced liver toxicity in Japanese quail. International Journal of AgriScience, 4: 362- 369.
Khosravinia H, Narasimha Murthy HN & Govindaiah MG. 2006. Imposing Restriction in Selection for Disproportionate Cut-Up Carcass Yield in an Experimental Flock of Broiler Chicken. The Journal of Poultry Science, 43: 109-119. DOI: 10.2141/jpsa.43.109
Kramer CY. 1956. Extension of multiple range tests to group means with unequal number of replications. Biometrics, 12: 307-310.
Lykkesfeldt J & Svendsen O. 2007. Oxidants and antioxidants in disease: oxidative stress in farm animals. Veterinary Journal, 173: 502-11. DOI: 10.1016/j.tvjl.2006.06.005
Manibusan M, Odin M & Eastmond D. 2007. Postulated carbon tetrachloride mode of action: A review. Journal of Environmental Sciences and Health part C Environmental Carcinogenesis and Ecotoxicology Reviews, 25: 185-209. DOI: 10.1080/10590500701569398
Moawad KM. 2007. Possible prophylactic effects of vitamin E or lycopene treatment on renal toxicity induced by CCl4 administration in albino rats. World Journal of Zoology, 2: 19-28.
Mohammadi Ghasem Abadi MH, Moravej H, Shivazad M, Karimi Torshizi MA & Kim WK. 2019. Effects of feed form and particle size, and pellet binder on performance, digestive tract parameters, intestinal morphology, and cecal microflora populations in broilers. Poultry Science, 98: 1432–1440. DOI: 10.3382/ps/pey488
Mokubedi ShM, Phoku JZ, Changwa RN, Gbashi S & Njobeh PB. 2019. Analysis of Mycotoxins Contamination in Poultry Feeds Manufactured in Selected Provinces of South Africa Using UHPLCMS/MS Sharon. Toxins, 11: 452. DOI: 10.3390/toxins11080452
Murugesan GR, Ledoux DR, Naehrer K, Berthiller F, Applegate TJ, Grenier B, Phillips TD & Schatzmayr G. 2015. Prevalence and effects of mycotoxins on poultry health and performance, and recent development in mycotoxin counteracting strategies. Poultry Science, 94: 1298–1315. DOI: 10.3382/ps/pev075
Nateghi R, Samadi F, Ganji F & Zerehdaran. S. 2013. Hepatoprotective effects of Cynara scolymus L. extract on CCl4 induced liver injury in broiler chickens. International Journal of AgriScience, 3: 678-688.
Nazar FN, Magnoli AP, Dalcero AM & Marin RH. 2012. Effect of feed contamination with aflatoxin B1 and administration of exogenous corticosterone on Japanese quail biochemical and immunological parameters. Poultry Science, 91: 47-54. DOI: 10.3382/ps.2011-01658
Nicolas G. 2019. Non-invasive diagnosis of liver steatosis: ready for primetime? Swiss Medical Weekly, 149. DOI: 10.4414/smw.2019.20108
Parmar MY, Shah PA, Thakkar VT, Al-Rejaie SS & Gandhi TR. 2012. Hepatoprotective effect of amomum subulatum roxb seeds on carbon tetrachloride-induced liver damage in rats. Journal of Pharamcy, 2: 38-43. DOI: 10.9790/3013- 25603843
Portillo-Sanchez P, Bril F, Maximos M, Lomonaco R, Biernacki D, Orsak B, Subbarayan S, Webb A, Hecht J & Cusi K. 2015. High prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus and normal plasma aminotransferase levels. Journal of Clinical Endocrinology and Metabolism, 100: 2231–2238. DOI: 10.1210/jc.2015-1966
Roll VFB, Lopes LL, Rossi P, Anciuti MA, Rutz F, Xavier EG & Silva SS. 2010. Hematology of broilers fed diets containing aflatoxins and mycotoxin adsorbent. Archivos de Zootecnia, 59: 93–101.
Sanjiv C. 2002. The Liver Book: A Comprehensive Guide to Diagnosis, Treatment and Recovery. Atria Company, Ohio, pp: 7.
SAS (Statistical Analysis System). 2003. SAS/STAT® 6. User's Guide. SAS Institute Inc. Cary, North Carolina.
Savard C, Tartaglione EV, Kuver R, Haigh WG, Farrell GC, Subramanian S, Chait A, Yeh MM, Quinn LS & Ioannou GN. 2013. Synergistic interaction of dietary cholesterol and dietary fat in inducing experimental steatohepatitis. Journal of Hepatology, 57: 81–92. DOI: 10.1002/hep.25789
Shirani A, Shivazad M, Samie A, Chamani M & Sadeghi AA. 2018. Effects of Starter Diet Feed Particle and Crumble Size on Performance, Carcass Characteristics and Small Intestinal Histomorphology in Broiler Chicks. Iranian Journal of Applied Animal Science, 8: 669-675.
Shuaib FM, Ehiri J, Abdullahi A, Williams JH & Jolly PE. 2010. Reproductive health effects of aflatoxins: a review of the literature. Reproductive Toxicology, 29: 262–270. DOI: 10.1016/j.reprotox
Sonkusale P, Bhandarker AG, Kurkare NV, Ravikanth K, Maini S & Sood D. 2011. Hepatoprotective activity of superliv liquid and repchol in CCl4 induced FLKS syndrome in broilers. International Journal of Poultry Science, 10, 49-55. DOI: 10.3923/ijps.2011.49.55
Takase T, Nakamura A, Miyoshi H, Yamamoto C & Atsumi T. 2017. Amelioration of fatty liver index in patients with type 2 diabetes on ipragliflozin: an association with glucose-lowering effects. Endocrine Journal, 64: 363–367. DOI: 10.1507/endocrj.EJ16-0295
Trott KA, Giannitti F, Rimoldi G, Hill A, Woods L, Barr B, Anderson M & Mete A. 2014. Fatty liver hemorrhagic syndrome in the backyard chicken. A Retrospective Histopathologic Case Series. Veterinary Pathology, 51: 787-795. DOI: 10.1177/0300985813503569
Vahed R, Kermanshahi H, Nasiri Moghaddam H,. Hassanabadi A & Beheshti Moghaddam S. 2016. Effect of different levels of marigold (calendula officinails) oil extract on performance, blood parameters and immune response of broiler chickens challenged with CCl4. Iranian Journal of Animal Science Research, 7: 447-455.
Wang F, Zuo Z, Chen K, Gao C, Yang Z, Zhao SD, Li J, Song H, Peng X, Fang J, Cui H, Ouyang P, Zhou Y, Shu G & Jing B. 2018. Histopathological injuries, ultrastructural changes, and depressed TLR expression in the small intestine of broiler chickens with aflatoxin B1. Toxins, 10: 131. DOI: 10.3390/toxins10040131
Yalcin A, Yumrutas O, Kuloglu T, Elibol E, Parlar A, Yilmaz I, Pehliva MN, Dogukan M, Uckardes F, Aydin H, Turk A, Uludag O, Sahin I, Ugur K & Aydin S. 2017. Hepatoprotective properties for Salvia cryptantha extract on carbon tetrachloride induced liver injury. Cellular and molecular biology, 63: 12. DOI: 10.14715/cmb/2017.63.12.13
Yoo W, Mayberry R, Bae S, Singh K, He QP, James W & Lillard JR. 2014. A Study of Effects of Multi Collinearity in the Multivariable Analysis. International Journal of Applied Science and Technology, 4: 9–19.
Zang JJ, Piao XS, Huang DS, Wang JJ, Ma X & Ma YX. 2009. Effects of feed particle size and feed form on growth performance, nutrient metabolizability and intestinal morphology in broiler chickens. Asian-Australasian Journal of Animal Sciences, 2: 107–112. DOI: 10.5713/ajas.2009.80352
Zhang Y, Liu Z, Liu R, Wang J, Zheng M, Li Q, Cui H, Zhao G & Wen j. 2018. Alteration of Hepatic Gene Expression along with the Inherited Phenotype of Acquired Fatty Liver in Chicken. Genes (Basel), 9: 199. DOI: 10.3390/genes9040199
Excellent. the liver is essential for many vital functions and for a correct biotransformation of nutrients to meat. Therefore, the safety of the raw material to be used is necessary. In addition, to know the metabolic routes through which the additives to be used will be metabolized in order to avoid overloading the liver. Many farms use liver protectors as a protocol, in order to strengthen this organ.














.jpg&w=3840&q=75)