I. INTRODUCTION
The quality of poultry feed and litter are important for maintaining and improving poultry production. Poultry feed (raw materials and formulated diet) is highly variable and large safety margins are needed to buffer the nutritional variability in feed ingredients when formulating diets (Moss et al., 2021). Feed ingredients delivered to feed mills are not comprehensively analysed using current methods (i.e., wet-chemistry and NIR). This is because the large quantities of feed being processed daily and the current methods rely on sampling and making assumptions that the ingredients are homogenised, which may not be the case. HSI can analyse a large quantity of material or an entire load, quantify the targeted properties, and display outputs in real-time. This allows automatic identification and quantification of impurities, moulds and mycotoxins, moist grains and nutritional values, while illustrating the variations for better management practices.
Litter moisture content (LMC) is another critical factor associated with concerns regarding environmental impacts, animal welfare, flock health, food safety and reductions in production efficiency (Carpenter et al., 1986; Dunlop et al., 2016; Lai et al., 2009). LMC varies in the shed due to many factors. Regular monitoring of LMC using the conventional methods in grower sheds is time-consuming and may produce inconsistent values. HSI can quantify and visualise LMC on a regular basis and potentially be combined with machine vision equipment in poultry sheds.
HSI cameras measure the light reflected from samples at different wavelengths in the visible to near infrared spectrum and uses artificial intelligence algorithms to correlate the targeted properties with the spectral information. HSI identifies different materials by using their spectral signatures (their reflectance/absorbance pattern), regardless of their colour and shape, and finds the relevant wavelength to quantify the concentrations.
In this study, we investigated the possibility of using HSI for quantifying the nutritional values of wheat as a feed ingredient, identifying impurities and wet grains in wheat, quantifying mycotoxins in wheat and quantifying LMC in different types of litter collected from a meat chicken shed during growing period.
II. METHOD
Wheat C and N concentrations 69 samples of wheat were collected from Queensland, New South Wales, Victoria, Western Australia, Tasmania and South Australia between 2016 and 2019. The samples were ground and analysed for carbon (C) and nitrogen (N) concentrations using a combustion analyser. The samples were then scanned using a visible-near infrared (VNIR) HSI camera (Resonon model Pika XC2, 400–1000 nm) and a shortwave infrared (SWIR) HSI camera (Hyspex model SWIR-384, 1000–2500 nm). Partial least square regression models (PLSR) were trained to correlate the concentrations of C and N with their spectral reflectance. Multiple PLSR models were trained to identify the most important spectral regions for predicting C and N concentrations using HSI (Tahmasbian et al., 2021). Models were tested using external (independent) test samples and evaluated using coefficient of determination (R2) and root mean square error (RMSE).
Wheat impurities: wheat samples from the previous stage were mixed with impurities including metal nuts, metal shavings, rubber, wood sticks and mouldy/moist grains. Samples were scanned using a SWIR HSI camera and partial least square discriminant analysis (PLS-DA) was trained to identify the spectral signatures. An independent test sample was used to evaluate the model and visualise the impurities.
Wheat mycotoxins: Wheat samples were artificially contaminated with different concentrations (0 to 10000 μg kg-1, added at 500 μg kg-1 intervals) of deoxynivalenol (DON), which is the most common mycotoxin in wheat. The samples were scanned using a SWIR HSI camera. The HSI data were correlated with the reference concentrations of DON using PLSR model. The model was tested using independent test samples.
Litter moisture content (LMC): Three types of chicken litter samples, including pine shavings, hardwood shavings and re-used hardwood were collected on multiple occasions during the growing period. Samples were dried and re-moistened to make moisture contents of 10%, 20%, 30%, 40%, 50% and saturated. Samples were scanned using the SWIR HSI camera inside plastic bags to maintain the LMC. The images of each sample varying in type, age and LMC were halved. The first half was used for training PLSR models to correlate the actual LMC with their spectral reflectance, while the other halves were used to evaluate the performance of the models. The models were initially trained by the entire spectra (950–2500 nm) to investigate and prove the possibilities of using HSI for litter moisture predictions and then a smaller spectral range (950–1000 nm) was used to investigate whether the prediction is possible using more affordable multispectral cameras, measuring 950–1000 nm only.
III. RESULTS
Wheat C and N concentrations: HSI combined with PLSR modelling predicted the concentrations of C and N in the ground wheat samples. The R2 of C prediction in the external
samples were 0.89 when the full VNIR spectral range (400–1000 nm) was used. The best spectral region for predicting C was 400–550 nm, being able to predict C concentrations with the R2 of 0.86.
HSI was also able to predict N concentrations in the samples with the R2 of 0.99 when using the full SWIR spectra (1000–2500 nm). VNIR HSI (full spectrum) was also able to predict the N concentrations with R2 of 0.91. While narrower spectral region in VNIR range could not predict the N concentrations, the best spectral regions for predicting N concentrations was 1451-1600 nm with R2 of 0.99.
Feed impurities: the PLS-DA model recognised and classified the pixels related to the normal wheat, metal nuts and shavings, rubber, wood sticks and mouldy/moist grains and displayed them in the images using different colours (Figure 1). The accuracy of the classification was > 90%.
Figure 1 - Identification of impurities and moist/mouldy grains in wheat using hyperspectral imaging
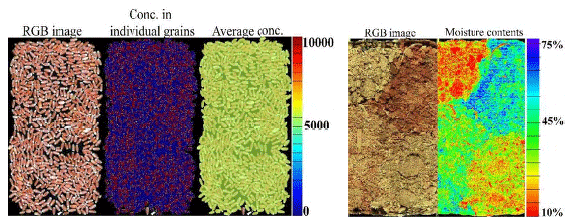
Feed mycotoxins: HSI was able to predict the concentrations of DON (0 to 10000 μg kg-1) in the external test samples (R2 of 0.97). The HSI was also able to display the concentration gradient of DON in the contaminated samples (Figure 2-left).
Figure 2 - Quantification of deoxynivalenol (μg/kg) in wheat samples (left) and moisture content of an array of four samples of chicken litter (re-used hardwood) ranging from 10-75% (right) using hyperspectral imaging (HSI, 950-2500 nm).
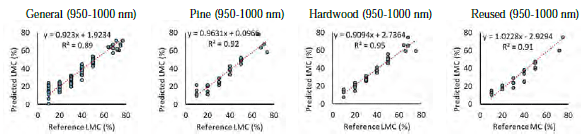
Litter moisture contents: HSI (950–2500 nm) combined with artificial intelligence predicted LMC in all litter type and age with R2 of 0.98 for the independent test samples. Reducing the spectral range to 950–1000 nm, reduced the R2 of the general model to 0.89 (Figure 3). Separating the samples based on litter type (pine, hardwood and re-used hardwood) increased the 950–1000 nm prediction R2 to over 0.91 for each litter type (Figure 3). The prediction algorithms were applied to a re-used hardwood litter sample to produce a gradient map of moisture content (Figure 2-right).
Figure 3 - Reference litter moisture content (LMC) versus the LCM predicted using general and separate partial least square models developed using 950-1000 nm wavelengths.
IV. DISCUSSION
HSI was successfully used to measure wheat C and N concentrations, impurities, mould and mycotoxins and litter moisture content in this study. Our results are consistent with and complementary to previous studies that used HSI for quantifying nutrients including calcium, magnesium, molybdenum, zinc and protein in wheat kernels and flour and detecting diseases including Fusarium infection (Bauriegel et al., 2011; Caporaso et al., 2018; Hu et al., 2021). HSI has potential to improve the accuracy of feed formulating by minimising or completely removing random/systematic sampling errors (a major source of inaccuracy) by scanning a large portion or the entire load of raw materials or formulated diet. This study was the first using HSI for predicting litter moisture content and we were unable to compare our results with previous studies.
HSI cameras are currently used mainly for research due to the high cost of the equipment (currently $80–250k depending on the spectral range and quality of the equipment). However, recognizing and using the important wavelengths only, such as what has been done in the present study, enables using cheaper multispectral cameras that can be used for different purposes (e.g., online monitoring of birds and measuring litter moisture) simultaneously.
Presented at the 34th Annual Australian Poultry Science Symposium 2023. For information on the next edition, click here. 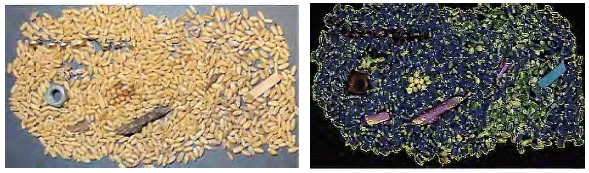









.jpg&w=3840&q=75)