Infectious Coryza
Published: June 23, 2016
By: María Luciana Cigoy, Yosef Huberman and Horacio Terzolo. Instituto Nacional de Tecnología Agropecuaria, Estación Experimental Balcarce (INTA EEA Balcarce), Argentina.
Introduction
Infectious Coryza is a highly contagious bacterial disease caused by Avibacterium paragallinarum (formerly called Haemophilus paragallinarum). It often affects the upper respiratory tract of chickens (Gallus gallus) but has been also described in quails and parrots. Birds of all ages are susceptible. The disease is well known in laying hens although often goes unnoticed in subclinically infected broilers due to difficulties of the standard bacteriological methods. On the other hand, it is important to differentiate infectious coryza caused by Avibacterium paragallinarum from “coryza of turkeys", which is completely different and is caused by Bordetella avium.
Production losses
The disease has worldwide distribution and it is mainly found in countries with intensive production where overcrowding and stress increase susceptibility of birds. Economic losses are produced due to a significant reduction in egg production (up to 40%) in layers or breeders after reaching peak production; weight loss in broilers (caused by diarrhoea and reduced consumption of water and food); and increased number of discarded chickens either at slaughter due to suffocation before being hung on the hook or due to dermatitis and cellulitis.
Pathogenicity and virulence of Avibacterium paragallinarum
The haemagglutinins (antigens that produce haemagglutination of red cells) of Avibacterium paragallinarum are structures mainly related to antigenicity, pathogenicity and immunogenicity. These haemagglutinins are antigens that agglutinate chicken red blood cells. In fact, these are adhesins that allow attachment of bacteria to structures on the mucosa cell surface and are considered essential for the first stage of infection. The chicken red blood cells share the same superficial receptors of the chicken epithelial cells of the respiratory tract mucosa. Thus, the active protection of an infected bird mainly depends on the presence of humoral antibodies, which inhibit the adhesion by neutralizing the hemagglutinin action. Other virulence factors, including the capsule in strains that cause septicaemia, could act by inhibiting phagocytosis and allowing distribution of the Avibacterium paragallinarum in other tissues.
Symptoms and lesions
Clinical signs include: nasal discharge, facial swelling with noticeable swelling of the face, lacrimation, anorexia, diarrhoea and it can also be hearing a snore or tracheal rattle when the lower respiratory tract is affected. Inflammation of the wattles is very rare but can occur in roosters.
According to clinical signs and head injuries, sick birds may be classified into four grades: Grade 1 (mild conjunctivitis); Grade 2 (periorbital swelling and sinus with or without conjunctivitis or tearing); Grade 3 (oedema and swelling of periorbital area and sinus, sinusitis, nasal and eye discharge, partial closure of the eye); Grade 4 (conjunctivitis with abundant nasal and eye discharge, severe periorbital swelling and sinus area, eyelids stuck and complete closure of eyes). Only those chickens having grades 2, 3 and 4 (Photos 1, 2 and 3) are considered to be sick.
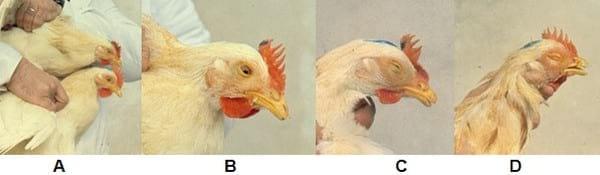
Photo 1: Grades in chickens experimentally inoculated and observed at the 2nd day post-infection.
A. Grade 0 (lower chicken) without any lesion and Grade 1 (top chicken) with mild conjunctivitis.
B. Grade 2. Conjunctivitis with partial closure of the eye and swelling of periorbital area and paranasal sinuses.
C. Grade 3. Conjunctivitis, eyelids closed but unbounded and obvious swelling of periorbital area and paranasal sinuses.
D. Grade 4. Conjunctivitis with eye completely closed, eyelids stuck and very severe swelling of the periorbital and sinus area.
A. Grade 0 (lower chicken) without any lesion and Grade 1 (top chicken) with mild conjunctivitis.
B. Grade 2. Conjunctivitis with partial closure of the eye and swelling of periorbital area and paranasal sinuses.
C. Grade 3. Conjunctivitis, eyelids closed but unbounded and obvious swelling of periorbital area and paranasal sinuses.
D. Grade 4. Conjunctivitis with eye completely closed, eyelids stuck and very severe swelling of the periorbital and sinus area.
Photos obtained from: SORIANO VARAS, E.; TERZOLO, H.R. Epizootiología, prevención y control de coriza infecciosa. Epizootiology, prevention and control of infectious coryza. Veterinaria México 35 (3): 272, 2004.

Photo 2. Infectious coryza in natural infection of hens. A. Grade 2. Note the slight swelling of the infraorbital sinus and abundant thick mucus when opening the peak. B. Grade 4. Note the severe swelling of the face, eyes completely closed with lids bounded, nasal and eye secretions and abundant mucus into the oral cavity.

Photo 3. Infectious coryza Grade 4 in a 35 day-old broiler experimentally inoculated and observed at the 2nd day post-infection. Note the severe swelling of the face, the abundant eye discharge with eyes completely closed and attached lids due to an intense inflammation.
Associated diseases
When Infectious coryza is associated with other viral or bacterial infectious agents such as infectious bronchitis virus, Mycoplasma gallisepticum, Avibacterium gallinarum (previously named Pasteurella gallinarum), Escherichia coli, Salmonella spp. or Pasteurella multocida, the disease worsens and prolongs its course and is denominated “Complicated Infectious Coryza " (Figure 1).
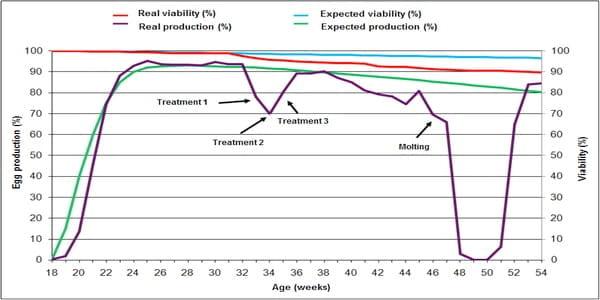
Figure 1. Comparison between the expected standard production of Lohmann Brown Classic line and the real production observed during a severe outbreak of Complicated Infectious Coryza associated with Mycoplasma gallisepticum and intestinal parasites in addition to bad farm management and poor biosecurity conditions. Note that the viability of hens is almost unaffected while the lay of eggs significantly decreased. Note the sharp fall of egg production from week 32 and the difficulty of maintaining it after successive antibiotic treatments (weeks 33, 34 and 35). On week 46 the food supply was suspended for 5 days to produce molting together with total absence of production, which restarted from week 51 onwards.
In broilers, Avibacterium paragallinarum may cause “swollen head syndrome" in which it is associated with other infectious agents, such as Escherichia coli, Bordetella avium, Mycoplasma gallisepticum and/or turkey rhinotracheitis (TRT) virus or infectious bronchitis virus, among others.
Poultry that suffer Complicated Infectious Coryza are not easily cured and usually the affected birds remain with several sequels, being common an increase in mortality and many discarded birds.
Duration of illness
When Avibacterium paragallinarum is not associated with other infectious agents, it has a short-term incubation period of 1 to 3 days and the symptoms persist for 3 to 7 days. In these uncomplicated cases, the disease is generally characterized by high morbidity and low mortality although it can also be asymptomatic without any mortality, or produce septicaemia and death, according to the virulence of the acting strain. However, when other bacterial or viral agents are associated with Avibacterium paragallinarum, the disease continues for several weeks (Figure 1).
Routes of infection and persistence of the pathogen in farms
The bacterium does not persist in the environment for a long period of time and therefore, the main reservoir of infection is the same sick birds. After recovering from the disease they continue to harbour Avibacterium paragallinarum but without showing any sign of disease. Despite these carrier birds remain apparently healthy, they are able to infect new susceptible young birds that are introduced into the farm. Hence, multi-age farms are not recommended.
In addition, Avibacterium paragallinarum can be introduced into free farms by air. For these reasons, biosecurity measures and proper distance between farms are very important to prevent entry of the pathogen, since once present on premises, eradication is very difficult, especially in multi-age farms.
Diagnosis
The clinical diagnosis of classical infectious coryza is relatively easy when it is not complicated with other infectious agents. However, even in these cases, it is very important to send samples to the laboratory to confirm the diagnosis and determine with certainty the acting serogroup and preserve strains that eventually might be used in vaccines adapted to the affected zone.
Sampling
When clinical signs of infectious coryza are observed, it is recommended to send three to five heads to a diagnostic laboratory. These heads should be selected from birds recently suffering from acute signs of the disease. It is important that heads are shipped frozen so that they remain as such throughout transportation until arrival at the laboratory.
Bacterial isolation is performed from mucus samples taken from the infraorbital sinus. This requires cauterizing the skin covering the infraorbital region using a red hot metal spatula heated with a Bunsen burner. Afterwards, a skin incision is practiced onto the cauterized area, the skin is separated and a sterile swab (Photo 4), previously moistened with sterile nutrient broth or phosphate buffered solution at slightly alkaline pH (7.2-7.4), is introduced through the incision into the infraorbital sinus. Another sampling technique involves cutting the upper beak behind the nostrils followed by the introduction of a sterile moistened swab, as mentioned above, to collect the mucus directly from inside the nasal turbinates. Additionally, in septicaemic cases, it is possible to isolate this bacterium from internal organs, such as lungs, air sacs, livers and spleens; in acute cases Avibacterium paragallinarum has been isolated even from inside eyeballs. Heads and organs may be transported frozen using dry ice.
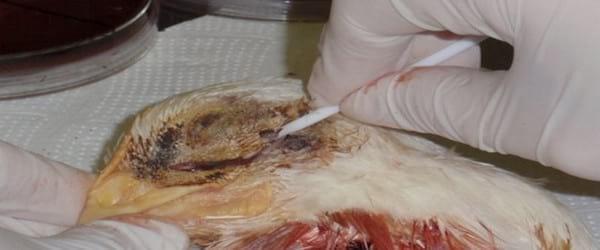
Photo 4. Swabbing the inside of the infraorbital sinus of a broiler. Prior to cutting the skin was cauterized to prevent contamination of the sample.
Isolation of Avibacterium paragallinarum
To be able to isolate this bacterium it is necessary to provide the intracellular coenzyme Nicotinamide Adenine Dinucleotide (NAD or V Factor) to the culture media, except for some independent strains that do not require free NAD in the culture medium.
Swab samples, taken from the infraorbital sinus can be streaked onto any rich nutrient agar plates (i.e. Columbia or Brain Heart Infusion) added with 7 per cent of whole desfibrinated (unhemolysed) blood previously seeded with a nurse strain, for instance Staphylococcus aureus. Staphylococcus aureus colonies haemolyses erythrocytes liberating free NAD, which diffuses to the nearby agar allowing a faint growth of Avibacterium paragallinarum. Hence, after incubation Avibacterium paragallinarum will grow as very small colonies surrounding the nurse strain and only over its haemolytic zone (Photo 5).
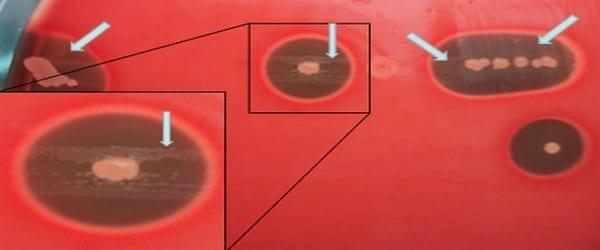
Photo 5. Development of Avibacterium paragallinarum on Columbia agar with addition of 7% (v/v) non-haemolysed bovine blood after 2 days of incubation period at 37°C. Avibacterium paragallinarum only develops around the colonies of Staphylococcus aureus (satellitism) and within the zone of hemolysis (arrows).
Alternatively, or additionally, the swab may be streaked on plates of any of the above mentioned rich nutrient agars adding 5 to 7 per cent of desfibrinated haemolysed horse blood. The blood may be haemolysed by heating at 56°C for 30-40 minutes into a water bath and afterwards added to the agar. Other preparation method is to haemolyse the blood by pouring it into a previously heated agar at 56°C into a water bath (chocolate agar). The heating process releases intracellular NAD from the erythrocytes and at the same time inactivates the growth inhibitors of the blood. Agars prepared with haemolysed blood do not require addition of the afore mentioned nurse strain. Besides, a selective agar may be prepared by adding bacitracin (5 IU/mL); cloxacillin (5 μg/mL) and vancomycin (20 μg/mL). In these selective or non-selective agars Avibacterium paragallinarum will grow as bright mucoid colonies (Photo 6).
Alternatively, or additionally, the swab may be streaked on plates of any of the above mentioned rich nutrient agars adding 5 to 7 per cent of desfibrinated haemolysed horse blood. The blood may be haemolysed by heating at 56°C for 30-40 minutes into a water bath and afterwards added to the agar. Other preparation method is to haemolyse the blood by pouring it into a previously heated agar at 56°C into a water bath (chocolate agar). The heating process releases intracellular NAD from the erythrocytes and at the same time inactivates the growth inhibitors of the blood. Agars prepared with haemolysed blood do not require addition of the afore mentioned nurse strain. Besides, a selective agar may be prepared by adding bacitracin (5 IU/mL); cloxacillin (5 μg/mL) and vancomycin (20 μg/mL). In these selective or non-selective agars Avibacterium paragallinarum will grow as bright mucoid colonies (Photo 6).

Photo 6. Abundant development of Avibacterium paragallinarum colonies in pure culture after 2 days of incubation period at 37°C onto agar Columbia with addition of 7% (v/v) hemolyzed horse blood. The haemolysis was produced by heating the blood into a water bath at 56°C for 35 minutes. Avibacterium paragallinarum develops across the whole plate as bright mucoid colonies.
Incubation is performed at 37 °C for 48 hours under a microaerophilic atmosphere obtainable by any of these procedures: the classical candle jar method; any CO2 incubator; replacement of 10 per cent of the air contained in a jar by carbon dioxide; or any commercial gas mixture for generating Campylobacter microaerophilic atmospheres.
Incubation is performed at 37 °C for 48 hours under a microaerophilic atmosphere obtainable by any of these procedures: the classical candle jar method; any CO2 incubator; replacement of 10 per cent of the air contained in a jar by carbon dioxide; or any commercial gas mixture for generating Campylobacter microaerophilic atmospheres.
As can be seen in Photo 5, using the unhemolysed blood agar Avibacterium paragallinarum grows very weakly and only around the nursing colonies (phenomenon called satelitism). Instead, as shown in Photo 6, using agar with the addition of lacquered or lysed blood, Avibacterium paragallinarum grows as large colonies covering the entire agar plate without depending on the feeding strain.
Isolates may be kept frozen in liquid nitrogen or at -80ºC by depositing (without disaggregation) a lump collected from the growth of a plate into a small 1-2 mL vial containing 0.5 mL of tryptose phosphate broth or brain-heart infusion added with 5 per cent (v/v) of decomplemented equine serum and 17 per cent (v/v) of tyndallized glycerol.
Serogroups, serovars and serological tests
According to the Page scheme, Avibacterium paragallinarum is classified into three serogroups, designated A, B and C. This serotyping is based on a haemagglutination inhibition (HI) test using rabbit antisera against A, B and C standard strains, fixed chicken red cells and the haemagglutinins of the strain to be classified. This HI test allows knowing which serogroup is present in an outbreak and could help make rapid decisions regarding which vaccine should be given.
The Kume scheme modified by Blackall recognised nine serovars distributed into the three Page serogroups: A-1, A-2, A-3, A-4, B-1, C-1, C-2, C-3 and C-4. This test is used to determine the circulating serovars and, therefore, may be used to define which serovar/s should be included in the bacterins administered in a particular region.
Several serological tests for detection of Avibacterium paragallinarum antibodies in chickens have been described: hemagglutination inhibition (HI), gel precipitation, agglutination, latex agglutination and ELISA.
Vaccination
Currently inactivated bivalent (serovars A-1 and C-1) or trivalent (A-1, B-1 and C-2) bacterins are used according to the incidence of serovars in different geographical regions. However, there have been described cases in which vaccines did not adequately protect, as happened with outbreaks caused by “variant” serovar B strains, which have very high pathogenicity. These B strains had to be added to the standard vaccine formulation marketed in some regions where they are present. Vaccines based on killed antigens have no cross protection between the three serogroups, so it is necessary to include those serogroups that are present in the geographical area to which the vaccine is intended to be used. Furthermore, challenge tests carried out between vaccinated and unvaccinated chickens showed that some serovars of the same serogroup (either B or C) have no cross protection among themselves. Therefore, in certain cases, application of autogenous bacterins or auto-vaccines, which include the strain present in the farm, is an effective preventive measure.
Vaccines are injected to the birds during rearing, usually before posture, and it is recommended to vaccinate subcutaneously into the dorsal region of the neck, in the distal area of the head, although vaccines can also be intramuscularly inoculated into the breast. Immunization of birds should be performed before 20 weeks of age, administering two doses separated by an interval of 3-4 weeks, considering that the last dose has to be administered about 3 weeks before hens start laying. On the other hand, it is recommended to revaccinate 10 days after completing the molting. In areas exposed to the disease, it is recommended to apply an extra initial dose at 5 weeks of age. Only in cases of outbreak risk during production, booster vaccinations in laying hens and breeders are recommended.
Complete protection in hens is achieved about 15-20 days after administration of the second dose and lasts between 11 and 14 months, according to the type and quality of the vaccine adjuvant. Available vaccines on the market are adjuvanted with aluminium hydroxide gel or double oil emulsions, among others.
Broilers are only exceptionally vaccinated when raised in areas heavily exposed to infection by administering a single dose at the hatchery or later at 15 or 20 days of age.
Treatment
Various chemotherapeutics and antibiotics have been administered to affected birds via drinking water, often combined with parenteral treatments. When spread of the disease is slow between rows of cages, the birds with symptoms must be injected and re-injected whereas administering medication to the whole flock through water or food. Some examples include the use of enrofloxacin at 10 mg/kg body weight or amoxicillin at 20 mg/kg for at least a week. Combinations of sulfachlorpyridazine-trimethoprim and sulfadimetoxine-trimethoprim via drinking water have also been used, but this administration should be carefully carried out because they may cause kidney damage in the birds.
Often, infectious coryza cases are complicated with Mycoplasma spp.; in such cases an example of a classical treatment that provides good results is the use of streptomycin at 100 mg/kg body weight together with tylosin (30 mg/kg), injecting the combination of both antibiotics subcutaneously. Other parenteral subcutaneous treatments for cases not associated with Mycoplasma spp. are enrofloxacin (20-25 mg/kg) or kanamycin (30 mg/kg) associated with gentamicin (5-8 mg/kg).
Although these treatments have been very successful, there has been an increase in bacterial resistance. For this reason, and considering that antimicrobial susceptibility varies, it is recommended to carry out sensitivity tests in order to select the most appropriate antimicrobial and define strategies for the use of drugs. The indiscriminate and repetitive use of antimicrobials, without testing the degree of sensitivity of the strains present in the farm, promotes bacterial resistance to antimicrobials reducing the effect of the treatment, which also results in unnecessary expenses.
Fumigation
It is important to fumigate (using coarse drops) over the chickens with quaternary ammonium compounds to prevent respiratory transmission, fumigating twice a day. It is important to note that spraying only works as a complementary measure in vaccinated birds but their action is of little help when birds have not been previously immunized.
It should be considered that, after treatment, the infection may be controlled but can never achieve a complete eradication of the disease from the farm, being very important the instauration of biosecurity and disinfection programs. Birds that have become sick, once recovered, act as healthy carriers, so it is highly recommended to treat the animals, perform susceptibility testing for future treatments on the farm and also immunise during rearing all new entering birds into the affected farm.
Acknowledgements
The authors thank Dr. Fernando Navarro (Veterinary Lazomar, Mar del Plata, Argentina) and Dr. Bernardo Kojic Rousseil (Granja Don Cosme, Buenos Aires, Argentina), for their contributions and technical comments.
Bibliography
1. ABD EL-GHANY, W. A. Evaluation of autogenous Avibacterium paragallinarum bacterins in chickens. International Journal of Poultry Science 10 (1): 56-61, 2011.
2. BLACKALL, P.J. Infectious coryza: Overview of the disease and new diagnostic options. Clinical Microbiological Reviews 12 (4): 627-632, 1999.
3. BLACKALL, P.J.; CHISTENSEN, H.; BECKENHAM, T.; BLACKALL, L.L.; BISGAARD, M. Reclassification of Pasteurella gallinarum, Haemophilus paragallinarum, Pasteurella avium and Pasteurella volantium as Avibacterium gallinarum gen. nov., comb. nov., Avibacterium paragallinarum comb. nov., Avibacterium avium comb. nov. and Avibacterium volantium comb. nov. International Journal Systematic and Evolutionary Microbiology 55: 353-362, 2005.
4. BLACKALL P.J.; EAVES L.E..; ROGERS D.G. Proposal of a new serovar and altered nomenclature for Haemophilus paragallinarum in the Kume Hemagglutinin Scheme. Journal of Clinical Microbiology 28 (6): 1185-1187, 1990.
5. BRAGG, R. R. Limitation of the spread and impact of infectious coryza through the use of continuous disinfection programme. Onderstepoort Journal of Veterinary Research 71: 1-8, 2004.
6. CONDE, M. D.; HUBERMAN, Y. D.; MENDOZA-ESPINOZA, A.; DELGADO, R. I.; TERZOLO H. R. Vaccination of one-day-old broiler chicks against Infectious Coryza. Avian Diseases 55 (1): 119-122, 2011 & Diseases Digest. 6 (1):e37-e38, 2011.
7. DROUAL, R.; BICKFORD, A.A.; CHARLON, B.R.; COOPER G.L.; CHANNING, S.E. Infectious coryza in meat chickens in the San Joaquin Valley of California. Avian Dis., 34: 1009-1016, 1990.
8. HUBERMAN, Y. D.; BUENO, D. J.; TERZOLO, H. R. Evaluation of the protection conferred by a disinfectant against clinical disease caused by Avibacterium paragallinarum serovars A, B and C from Argentina. Avian Diseases 49 (3): 588-591, 2005.
9. JACOBS, A. A.; CUENEN, W.; STORM, P. K. Efficacy of a trivalent Haemophilus paragallinarum vaccine compared to bivalent vaccines. Veterinary Microbiology 32 (1): 43-49, 1992.
10. MENDOZA-ESPINOZA, A.; TERZOLO, H. R.; DELGADO, R. I.; ZAVALETA, A. I.; KOGA, Y. HUBERMAN, Y. D. Serotyping of Avibacterium paragallinarum isolates from Peru. Avian Diseases 53 (3): 462-465, 2009 & Avian Diseases Digest 4 (3): e22, 2009.
11. SANDOVAL, V. E.; TERZOLO H. R.; BLACKALL P. J. Complicated infectious coryza outbreaks in Argentina. Avian Diseases 38: 672-678, 1994.
12. SORIANO VARAS, E.; LOGINOS GARDUÑO, M.; TÉLLEZ, G.; POMPOSO FERNÁNDEZ, R.; SUÁREZ GÜEMEZ, F.; BLACKALL, P.J. Cross-protection study of the nine serovars of Haemophilus paragallinarum in the Kume haemagglutinin scheme. Avian Pathology 33 (5): 506-511, 2004.
13. SORIANO VARAS, E.; TERZOLO, H.R. Haemophilus paragallinarum: Etiology of infectious coryza. Veterinaria México 35 (3): 245-259, 2004.
14. SORIANO VARAS, E.; TERZOLO, H.R. Epizootiology, prevention and control of infectious coryza. Veterinaria México 35 (3): 261-279, 2004.
15. TERZOLO, H. R.; SANDOVAL, V. E.; GONZÁLEZ PONDAL, F. Evaluation of inactivated infectious coryza vaccines in chickens challenged by serovar B strains of Haemophilus paragallinarum. Avian Pathology 26: 365-376, 1997.
16. TERZOLO, H.R.; PAOLICCHI, F.A.; SANDOVAL, V.E.; BLACKALL, P.J.; YAMAGUCHI, T.; IRITANI, Y. Characterization of isolates of Haemophilus paragallinarum from Argentina. Avian Diseases 37: 310-314, 1993.
2. BLACKALL, P.J. Infectious coryza: Overview of the disease and new diagnostic options. Clinical Microbiological Reviews 12 (4): 627-632, 1999.
3. BLACKALL, P.J.; CHISTENSEN, H.; BECKENHAM, T.; BLACKALL, L.L.; BISGAARD, M. Reclassification of Pasteurella gallinarum, Haemophilus paragallinarum, Pasteurella avium and Pasteurella volantium as Avibacterium gallinarum gen. nov., comb. nov., Avibacterium paragallinarum comb. nov., Avibacterium avium comb. nov. and Avibacterium volantium comb. nov. International Journal Systematic and Evolutionary Microbiology 55: 353-362, 2005.
4. BLACKALL P.J.; EAVES L.E..; ROGERS D.G. Proposal of a new serovar and altered nomenclature for Haemophilus paragallinarum in the Kume Hemagglutinin Scheme. Journal of Clinical Microbiology 28 (6): 1185-1187, 1990.
5. BRAGG, R. R. Limitation of the spread and impact of infectious coryza through the use of continuous disinfection programme. Onderstepoort Journal of Veterinary Research 71: 1-8, 2004.
6. CONDE, M. D.; HUBERMAN, Y. D.; MENDOZA-ESPINOZA, A.; DELGADO, R. I.; TERZOLO H. R. Vaccination of one-day-old broiler chicks against Infectious Coryza. Avian Diseases 55 (1): 119-122, 2011 & Diseases Digest. 6 (1):e37-e38, 2011.
7. DROUAL, R.; BICKFORD, A.A.; CHARLON, B.R.; COOPER G.L.; CHANNING, S.E. Infectious coryza in meat chickens in the San Joaquin Valley of California. Avian Dis., 34: 1009-1016, 1990.
8. HUBERMAN, Y. D.; BUENO, D. J.; TERZOLO, H. R. Evaluation of the protection conferred by a disinfectant against clinical disease caused by Avibacterium paragallinarum serovars A, B and C from Argentina. Avian Diseases 49 (3): 588-591, 2005.
9. JACOBS, A. A.; CUENEN, W.; STORM, P. K. Efficacy of a trivalent Haemophilus paragallinarum vaccine compared to bivalent vaccines. Veterinary Microbiology 32 (1): 43-49, 1992.
10. MENDOZA-ESPINOZA, A.; TERZOLO, H. R.; DELGADO, R. I.; ZAVALETA, A. I.; KOGA, Y. HUBERMAN, Y. D. Serotyping of Avibacterium paragallinarum isolates from Peru. Avian Diseases 53 (3): 462-465, 2009 & Avian Diseases Digest 4 (3): e22, 2009.
11. SANDOVAL, V. E.; TERZOLO H. R.; BLACKALL P. J. Complicated infectious coryza outbreaks in Argentina. Avian Diseases 38: 672-678, 1994.
12. SORIANO VARAS, E.; LOGINOS GARDUÑO, M.; TÉLLEZ, G.; POMPOSO FERNÁNDEZ, R.; SUÁREZ GÜEMEZ, F.; BLACKALL, P.J. Cross-protection study of the nine serovars of Haemophilus paragallinarum in the Kume haemagglutinin scheme. Avian Pathology 33 (5): 506-511, 2004.
13. SORIANO VARAS, E.; TERZOLO, H.R. Haemophilus paragallinarum: Etiology of infectious coryza. Veterinaria México 35 (3): 245-259, 2004.
14. SORIANO VARAS, E.; TERZOLO, H.R. Epizootiology, prevention and control of infectious coryza. Veterinaria México 35 (3): 261-279, 2004.
15. TERZOLO, H. R.; SANDOVAL, V. E.; GONZÁLEZ PONDAL, F. Evaluation of inactivated infectious coryza vaccines in chickens challenged by serovar B strains of Haemophilus paragallinarum. Avian Pathology 26: 365-376, 1997.
16. TERZOLO, H.R.; PAOLICCHI, F.A.; SANDOVAL, V.E.; BLACKALL, P.J.; YAMAGUCHI, T.; IRITANI, Y. Characterization of isolates of Haemophilus paragallinarum from Argentina. Avian Diseases 37: 310-314, 1993.
Note
A similar summarized article was published (in Spanish) by the authors in:
Related topics:
Authors:


Influencers who recommended :
Ozlem SAHAN YAPICIERRecommend
Comment
Share

11 de diciembre de 2021
If infectious coryza attacks a flock in early growing stage (say below 10 weeks) the treatment with antibiotics is enough. The disease spreads fast and the flock becomes immune for life. The flock does not require vaccination. Prompt treatment and biosecurity keeps the mortality under control.
If the disease occurs on a multiage group farm for the first time where the vaccination was not being practiced, better go for the vaccination for the healthy flocks and tighten the biosecurity. The damage to the farm will be less.
Recommend
Reply

16 de agosto de 2016
Dear Vihang Patil,
Thank you for your comments about our work and your queries regarding vaccination!
I would like to say that in some parts of the world vaccines do not contain serovar B, simply because this serovar does not exist in this geographical areas. Regarding the expression standard strains, we should not include some standard strains that are used routinely used for serological classification because this are “laboratory strains” subjected to multiple subcultures that make this strains to loss pathogenicity together with antigenicity. In fact, you can even isolate (from apparently healthy flocks) field strains that are unclassifiable due to loss of haemagglutination antigens and are totally apathogenic. The correct antigen for a vaccine should include strains able to cause disease and hence also able to be evaluated in their protection by challenge- protection trials when confronted against vaccinated and unvaccinated chickens.
Regarding adjuvants, it depends upon the quality of the adjuvant. Some commercial vaccines including oil adjuvant are excellent. The double emulsion oil is the only adequate for poultry; other simple emulsion used in another animal species are not suitable. Nevertheless, for preparing auto-vaccines of limited use in some areas it is easier to prepare them using a good quality aluminum hydroxide gel; the duration of the protection depends upon the quality of the gel, there are some interesting papers on this subjects (see Blackall et al.).
Best regards,
Dr. Horacio Raúl Terzolo
Recommend
Reply

15 de agosto de 2016
Dear William T H Chang,
Interesting observation of your research.
Could you please give us more details regarding the mechanism of action of beta-glucan from yeast cell wall acting in any infection (bacterial?, viral?)
Do you think that this feed ingredient could be active against the Avibacterium paragallinarum?
There is some experience on infectious coryza?
Thank you!
Best regards,,
Dr. Horacio Raúl Terzolo
Recommend
Reply
13 de agosto de 2016
Infectious Coryza is a kind of infection that spreads very fast within the flock . Although, vaccination is the best method of prevention ,but control must be looked at from the angle of strict sanitation and hygiene. Poultry managers and attendants should be observant to cull birds that shows first signs/ symptoms of the infection .
Recommend
Reply
Fusion Biosystems
12 de agosto de 2016
Hi Horacio, there is a management dimension Infectious Coryza persists in production areas where hens are not culled on time. So it is important not to keep too old layers or to have birds of different age close by during production. Although the latter measure is difficult for infrastructural reasons, but it is professionally better if we have rearing operations far separated from layer egg segment.
Recommend
Reply

11 de agosto de 2016
Dr.Talapaneni.Kotaiah,
When vaccine failures occur prevention is not possible anymore and treatment with antibiotics are indicated until new preventive or corrective measures are implemented.
Best regards,
Dr. Horacio Raúl Terzolo
Recommend
Reply

11 de agosto de 2016
Dear Vihang Patil,
Thank you for your positive opinion about the usefulness of this article!
I agree completely that vaccination with dead autogenous bacterins (representative of the antigens acting in the geographical area) is the best preventive way together with biosecurity and hygienic management with additional prevention targeted against of other infectious agents, particularly Mycoplasma spp. and respiratory viruses such as for example infectious bronchitis.
Your very interesting work about the variation of the sero-prevalence points out the diversity of bacterial antigens within a same microorganism and the strong possibility of vaccine failures due to development of new strains that replace the existent which are inhibited by the immune-protection of the vaccinated birds against the previous strains. This fact has been proved for infectious coryza when serovar B caused extensive outbreaks in hens which were only vaccinated against serovars A and C, as at that time it was wrongly believed that this serovar B was non-pathogenic or non-existing anymore despite it has been described by previous researchers. Also, if the standard commercial dead vaccine adequately protects the birds it would not be necessary to administer autogenous vaccinations.
Best regards,
Dr. Horacio Raúl Terzolo
Recommend
Reply

3 de agosto de 2016
what will you do if the disease occurs?
killed vaccines will nor help treat your birds
Recommend
Reply

8 de julio de 2016
Dear All,
All your comments are very useful and enrich the discussion!
We also have to bear in mind the fact that diseases usually come associated with other bacteria and viruses. Mycoplasma is frequently associated but not always present. Some very pathogenic strains are able to cause severe disease per se, even with septicaemia and infections of the lower respiratory tract, that is our experience of serovar B in Argentina. We also found that sometimes Avibacterium gallinarum (ex- Pasteurella gallinarum) usually comes after the outbreak of Coryza severely affecting the eyes and paranasal sinuses with development of caseous material, causing loss of the sight in many hens. Have you had the same clinical picture?
Dr. Horacio Raúl Terzolo
Recommend
Reply

8 de julio de 2016
Dear Bouayard,
Thank you for sharing your experience with the disease!
Surely, effective vaccination with the predominant strains in the geographical area in where the farm is located is a good preventive measure coupled with strict biosecurity measures and disinfection. If the disease is present enrofloxacin may be a good treatment to be implemented, although sensitivity tests are always recommended to avoid selection of resistant strains.
Best regards,
Dr. Horacio Raúl Terzolo
Recommend
Reply

Would you like to discuss another topic? Create a new post to engage with experts in the community.
Featured users in Poultry Industry

Shivaram Rao
Pilgrim´s
PhD Director Principal de Nutrición y Servicios Técnicos de Pilgrim’s Pride Corporation
United States
United States








.jpg&w=3840&q=75)






