ABSTRACT
The effect of dietary inclusion of Bacillus subtilis DSM 32315 on the intestinal health and growth performance of Cobb 500 male broilers subjected to a Clostridium perfringens-induced necrotic enteritis (NE) challenge was determined in 2 experiments. In experiment 1, chicks were randomly assigned to 4 treatments of 10 replicate/treatment. In experiment 2, chicks were randomly assigned to 4 treatments of 12 replicates/treatment. The experimental treatments were non-infected, non-supplemented control, infected, non-supplemented control (IC), infected + Bacillus subtilis DSM 32315 (B. subtilis DSM 32315), infected + bacitracin methylene disalicylate (BMD). In both experiments, NE was induced by oral inoculation of toxin producing C. perfringens on 3 consecutive days between 17 and 20 D of age, following exposure of birds to predisposing conditions. At day 28 (experiment 1), broilers fed diets with B. subtilis DSM 32315 exhibited a significantly higher body weight, lower mortality, and intestinal NE lesion score, compared to the IC treatment.
At day 42 (experiment 2), B. subtilis DSM 32315 supplementation significantly improved BW, feed conversion ratio, production efficiency factor, NE lesion score, and mortality, compared to IC treatment. The effect of B. subtilis DSM 32315 on intestinal integrity of NE challenged chickens was evaluated with histomorphometry. A significantly shallower crypt depth and higher villus height to crypt depth ratio were observed in the mid-intestine of birds belonging to the B. subtilis DSM 32315 group, compared to the IC group. Furthermore, B. subtilis DSM 32315 supplementation significantly reduced the enteritis index associated with NE. In both experiments, the effect of B. subtilis DSM 32315 on the phenotypic measurements of NE and performance was comparable to the effect observed with BMD supplementation. In conclusion, supplementation of the direct fed microbial strain B. subtilis DSM 32315 can ameliorate the pathology and performance detriments associated with NE.
Key words:Bacillus subtilis, broiler, Clostridium perfringens, performance, necrotic enteritis.
INTRODUCTION
The world population is predicted to increase to over 9 billion by 2050, creating a challenge of securing adequate food supply for the growing population (Tomlinson, 2013). Animal protein is important for meeting the demand for macronutrient protein, an integral part of a balanced diet. The FAO projects that by 2020, chicken will be the world’s most consumed animal protein source by weight (Henchion et al., 2017). This may be because poultry production being cost-effective and resource-efficient is more affordable by consumers. However, a global growing consumer demand to eliminate or reduce antibiotics use due to the risk of antibiotic resistance has resulted in the re-emergence of some primary and/or secondary intestinal diseases resulting from the outgrowth of opportunistic pathogens, particularly Clostridium perfringens (Ratcliff, 2000; Shane, 2004; Van Immerseel et al., 2004; Singer and WilliamsNguyen, 2014).
Clostridium perfringens is a Gram-positive, sporeforming anaerobic bacterium that inhabits the chicken’s normal intestinal tract (Opengart, 2008). Certain predisposing factors, such as Eimeria spp. infections, high crude protein diets, or diets rich in non-starch polysaccharides, allow for the outgrowth of toxin producing (e.g., α- toxin, β-toxin, and netB-toxin) C. perfringens type A and type C strains. The resulting damage to the small intestine epithelium is the characteristic necrotic lesions of the disease necrotic enteritis (NE; Opengart, 2008; Timbermont et al., 2011; Paiva and McElroy, 2014). Research-driven strategies to control NE without the use of antibiotic growth promoters (AGP) or therapeutic antibiotic treatment have focused on feed additives or dietary modifications that enhance intestinal health. Candidate non-antibiotic feed additives such as probiotics, prebiotics, and organic acids have been shown to have potential to control NE and improve intestinal health (Hofacre et al., 2003; Dahiya et al., 2006; Ducatelle et al., 2015; M’Sadeq et al., 2015). Probiotics, also known as direct fed microbials (DFM), have been well studied as non-antibiotic feed additives (alternatives to AGP) due to their potential to control intestinal pathogens through several potential modes of action, such as competition with pathogens for nutrient and adhesions (competitive exclusion), increased digestive enzyme activity, production of substances that can inhibit the growth of pathogens or neutralize enterotoxins, modulation of the host’s immune development, and alteration of intestinal microbial activity (Dahiya et al., 2006; Schoster et al., 2013; Caly et al., 2015; M’Sadeq et al., 2015).
Several studies have examined the activity of different strains of the Bacillus spp. to reduce C. perfringens and improve the health and performance detriments of NE in broiler chickens (Murry et al., 2004; Jayaraman et al., 2013; Schoster et al., 2013; Whelan et al., 2018). Bacillus is a Gram-positive, rod-shaped, spore-forming, aerobic, or facultative anaerobic genus of bacteria. Bacillus-based probiotics are favored for infeed application because they offer better heat stability and higher acid tolerance, which are important for production of pelleted poultry feed and the acidic environment of the chickens’ gastrointestinal tract, respectively (Elshaghabee et al., 2017). Each strain of Bacillus spp. has unique characteristics that help define its activity, function, and potential benefits to the host animal. Therefore, individual Bacillus strains need to be evaluated for safety, stability in feed production, and efficacy in the host (Elshaghabee et al., 2017). Bacillus subtilis DSM 32315 is a naturally occurring strain shown to have in vitro inhibitory activity against toxin producing C. perfringens, through its inherent potential to express different secondary metabolites (unpublished data). In addition, previous in vivo study has shown that it can support the reduction of C. perfringens in the ileum and cecum, positively alter the intestinal microbial population, and support the improvement of growth performance (Whelan et al., 2018; Bortoluzzi et al., 2019). Based on these, it was hypothesized that Bacillus subtilis DSM 32315 can be an effective non-antibiotic feed additive to control NE and improve the performance of broiler chicken subjected to NE challenge.
These current studies were conducted to investigate whether supplementation of B. subtilis DSM 32315 spores in broiler chicken feed could prevent the performance detriments associated with NE in a comparable manner to the AGP, bacitracin methylene disalicylate (BMD). In addition, we examined whether the supplementation of B. subtilis DSM 32315 spores affected the intestinal architecture of broiler chickens challenged with NE.
MATERIALS AND METHODS
To investigate the effect of B. subtilis DSM 32315 on NE-challenged broiler chickens, 2 experiments were conducted at 2 independent research facilities. Experiment 1 was a cage study conducted at Southern Poultry Research Inc. (Athens, GA). Experiment 2 was a floor pen study conducted at Virginia Diversified Research Corp (Harrisonburg, VA). The experiments were conducted in accordance with the Guide for the Care and Use of Agricultural Animals in Research and Teaching. All procedures were supervised by an attending veterinarian.
Experiment 1
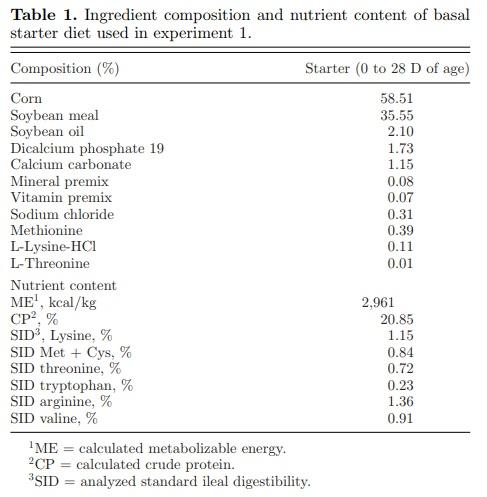
Animals and Diets A total of 320 one-day-old Cobb 500 male broiler chicks were randomly assigned to 4 dietary treatments consisting of 10 replicates per treatment and 8 birds per cage replicate, with cage as an experimental unit. A non-medicated (no antibiotic or anticoccidials drug), corn-soybean meal-based diet was used as the basal diet for all treatments (Table 1). Dietary treatments were then produced by supplementing the basal diet with test feed additive. The treatment groups were as follows: 1) non-infected, non-supplemented control (NC); 2) infected control, non-supplemented control (IC); 3) IC + Bacillus subtilis DSM 32315 at 1 × 106 CFU/g of feed (B. subtilis DSM 32315); and IC + 50 mg/kg of BMD. The single-phase mash diet was fed throughout 28 D of the study. Feed and water were provided ad libitum to all the birds, and all birds were reared under the same house conditions. Three feed samples were collected, one each from the beginning, middle, and end of the batch of treatment diet and mixed to form a composite sample. One sample was then obtained from the composite for each treatment and was used for Bacillus spore count analysis. The total spore count analysis was done by the dilution plate series culture method. Spore counts are reported as the log10 transformation of colony forming units per gram of feed (Log10 CFU/g feed; VDLUFA, 2012).
Necrotic Enteritis Challenge A NetB-positive C. perfringens (Hofacre et al., 1998) was used to induce the NE challenge. The challenge strain was inoculated into 1 L of thioglycolate broth supplemented with 5% beef extract and incubated at 37?C for 15 h. At day 13, all birds were orally inoculated with approximately 5,000 Eimeria maxima oocysts per bird. Beginning on day 18, and once daily for 3 consecutive days (days 18, 19, and 20), all birds except the NC group were administered a fresh broth culture of C. perfringens (approximately 1 × 108 CFU/mL) by oral gavage.
Performance Evaluation and NE Lesion Score Live body weight (BW) and feed intake were recorded on days 0, 13, 20, and 28 on cage basis to calculate body weight gain (BWG) and feed conversion ratio. Mortality was monitored and recorded each day. In addition, birds that were euthanized for tissue sampling were accounted as mortality and were individually weighed and recorded for calculating the mortality adjusted feed conversion ratio (aFCR). BW was reported as averages per bird. The aFCR from day 0 to 28 was reported on a cage basis, and the cumulative mortality from day 0 to 28 calculated as a percentage of cage was reported as average of all cage replicates in each treatment group. On day 20, 3 birds from each cage were selected, euthanized, weighed, and examined for the degree of presence of NE lesions in the mid intestine. The scoring was based on a 0 to 3 scale, with 0 representing normal tissue and 3 representing the presence of severe hemorrhagic intestinal lesions (Opengart, 2008).
Morphometry and Histology of Intestinal Tissues To assess the intestinal health of birds under the NE challenge, an intestinal histomorphometric evaluation was conducted. On day 20 (day of lesion scoring), the duodenum, mid-intestine, and cecum from 2 birds per cage were collected and placed in neutral-buffered formalin for histomorphometric evaluation. Tissue sample processing and histomorphometry were performed without knowledge of the treatment groups. The basis of the scoring methodology used in this study has been described by Hoerr and Schrader (2016). However, the description of the changes associated with each score is currently unpublished. Two-millimeter sections of each tissue were trimmed and placed in cassettes, processed for paraffin-embedded 5 μm sections, and stained with hematoxylin and eosin. All intestinal sections were kept intact in circular form to ensure uniformity of assessment. For each intestinal segment per bird, 5 villi height, crypt depth, and mucosal thickness readings were obtained, and the mean was calculated and reported. All the slides were evaluated using a Meiji MT5300L microscope with Canon Rebel T3i, 17.9MP camera. The slides were digitalized using the Image J software calibrated with AmScopeMR400.
For the histology, a lesion panel score was developed for each intestinal segment, and lesions were semiquantitatively scored for severity as follows: 0, normal; 1, minimal severity; 2, mild severity; 3, moderate; 4, marked; and 5, severe. Once the intestinal tissues were scored, the scores were then summed up to achieve an index score. The small intestine enteritis index (EI) was based on individual scoring for crypt hyperplasia, cystic crypts, villus shortening, lamina propria lymphocytes and plasma cells, dysbacteriosis, increased goblet cells and/or mucus, attenuated enterocytes, misshapen villus tips, and heterophils in the lamina propria. The ceca EI was based on scoring for lamina propria lymphoid hyperplasia, bacteria adherence to mucosa, cystic glands, lamina propria apoptosis, heterophils inflammation, necrosis, and increased intraepithelial leukocytes.
In addition, the duodenum and mid-intestine were scored for the degree of presence of E. maxima. The identification and scoring for the intestinal coccidia Index (CI) were performed as follows: E. maxima per entire gut section was scored as 0, no coccidia observed; 1, 0–20 coccidia; 2, up to 50 coccidia; 3, up to 75 coccidia; 4, up to 100 coccidia; 5, >100 coccidia. For each bird, the CI was calculated by summing the coccidia scores from each section of intestine. A total lesion index (TLI) was calculated by summing all lesion scores (EI and CI) for each section of intestine. A correlation analysis of macroscopic pathology (NE lesion scores at day 20) and microscopic pathology (histological scores at day 20), was conducted to compare the 2 methods.
Experiment 2
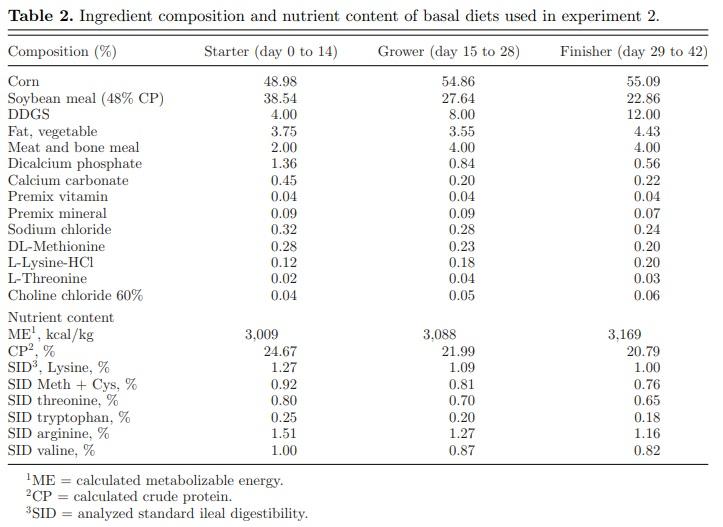
Animals and DietsA total of 1,440 day-old Cobb 500 male broiler chick were randomly assigned to 4 dietary treatments consisting of 12 replicates per treatment and 30 birds per pen replicate, with pen as the experimental unit. A basal diet primarily composed of corn, soybean meal, corn DDGS, and meat and bone meal (Table 2) was used for the starter (day 1 to 14), grower (day 15 to 28), and finisher (day 29 to 42) phases. Dietary treatments were then produced by supplementing the basal diet with test feed additive: 1) non-infected, non-supplemented control (NC); 2) infected control, non-supplemented control (IC); 3) IC + Bacillus subtilis DSM 32,315 at 1 × 106 CFU/g of feed (B. subtilis DSM 32315) and 4) infected + 50 g/MT of BMD. The study was conducted during the winter months beginning from December to February. Birds were reared on clean litter (wood shavings) which was top-dressed with used litter. Feed and water were provided ad libitum to all the birds.
Necrotic Enteritis Challenge On day 4, birds in each pen were exposed to approximately 2 kg of used litter that was sourced from healthy chickens that were not exposed to dietary DFM or enzymes. A pathogenic ield strain of C. perfringens that was isolated from a commercial poultry operation diagnosed with NE was used to induce the NE challenge. Fresh challenge inoculant was prepared prior to each challenge day; the C. perfringens isolate was incubated in cooked meat broth for approximately 24 h, and then transferred into sterile nutrient broth and incubated for approximately 18 h. On 3 consecutive days (days 17, 18, and 19), fresh broth culture of C. perfringens (approximately 1 × 109 CFU/mL) was individually administered to each infected bird by oral gavage.
Performance Evaluation and NE Lesion Score All birds and feeds were weighed on days 0, 14, 28, and 42. Feed consumption and BW were recorded on pen basis for each treatment group. BW and aFCR were reported as average per bird. Mortality was recorded daily, calculated as a percentage for each pen replicate, and reported as average of all replicate pens in each treatment. European Poultry Efficiency Factor (EPEF) values were calculated for overall growth period using the following standard formula: EPEF = [(BW in Kg x % Livability)/(Age in days x FCR)] x100. On day 21,3 birds per pen (36 birds per treatment) were randomly selected and evaluated for macroscopic intestinal NE lesions. Lesion scores were based on a 0 to 3 scoring system, with 0 being normal and 3 being the most severe (Opengart, 2008).
Statistical Analysis A randomized complete block design was used in both experiments. Data were analyzed using SAS software v9.3 (SAS Institute, 2012). The MIXED procedure was used for 1-way ANOVA with least square difference (LSD) means as a post hoc test for treatment comparison of BW, aFCR, EPEF, intestinal morphometry, and mortality.
Percentage mortality was arcsine transformed prior to ANOVA analysis. Necrotic enteritis lesion scores and histology scores were statistically analyzed using nonparametric Kruskal-Wallis test, with Dunn’s test nonparametric pairwise multiple comparisons as a post hoc test for treatment comparison. Spearman’s rank correlation coefficient (rho) was used for non-parametric correlation analysis of data obtained from the intestinal macroscopic and microscopic evaluation. Global and LSD means differences were considered significant at P ≤ 0.05.
RESULTS
Experiment 1
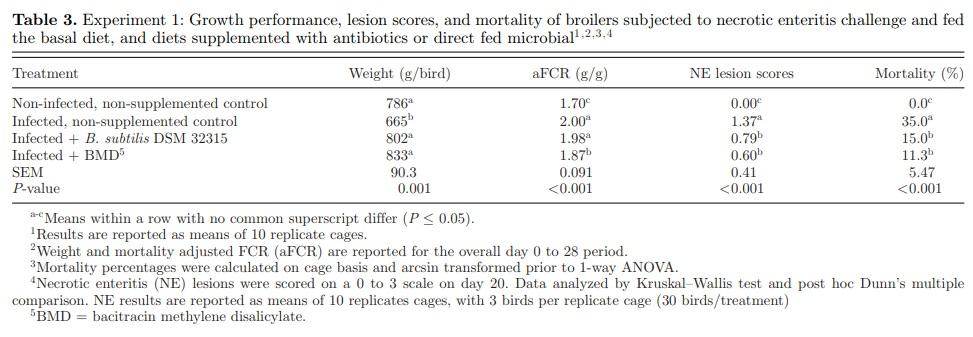
The results of spore count analysis of feed samples confirmed that B. subtilis DSM 32315 spores were supplemented at the expected target levels in all feeds (1 × 106 CFU/g feed). Day 0 to 28 growth performance, mortality, and intestinal lesion scores are presented in Table 3. The NE challenge model utilized in this study resulted in a significantly lower BW (665 g vs. 786 g) and higher aFCR (2.00 vs. 1.70) of birds in the IC treatment compared to the NC treatment. Furthermore, the cumulative mortality (35% vs. 0%) and mean NE lesion score (1.37 vs. 0) were significantly higher in the IC treatment compared to the NC treatment. The reduction in BW in the IC treatment was significantly improved (P = 0.001) by the supplementation of B. subtilis DSM 32315 (+ 137 g; 21%) and BMD (+168 g; 25%). The aFCR was improved in the BMD-treated group compared to the IC treatment group but was unaffected by B. subtilis DSM 32315 supplementation compared to the IC treatment group. The cumulative mortality (15% vs. 35%) and mean NE lesion score (0.79 vs. 1.37) were significantly improved (P <0.001) by the supplementation of B. subtilis DSM 32315, compared to the IC treatment, and this improvement was not significantly different from the BMD treatment.
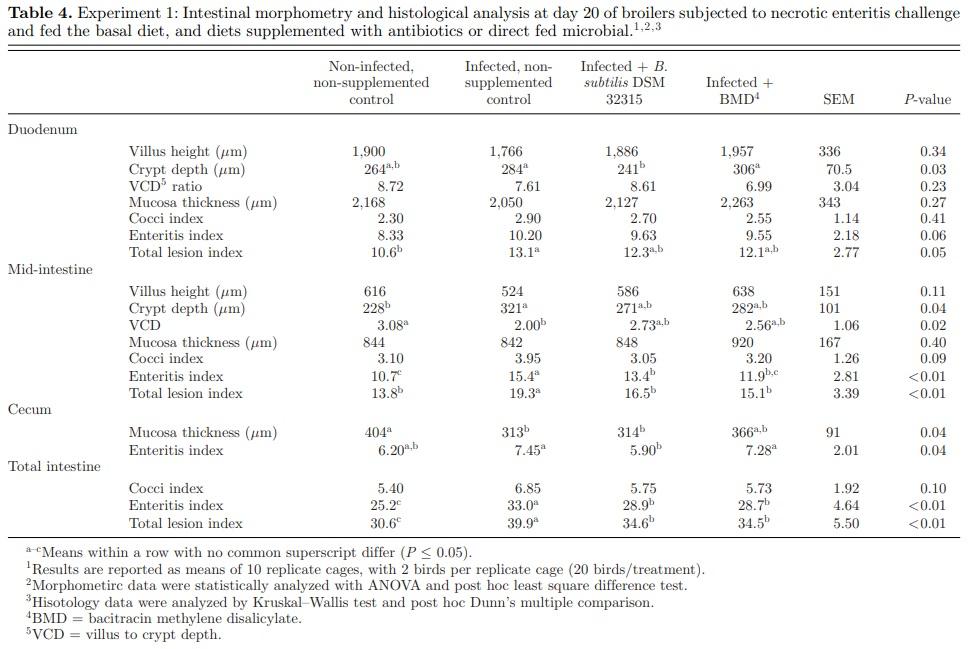
The results of histomorphometric analysis of the duodenum, mid-intestine, and cecum sampled on days 20 are presented in Table 4. There was a significant effect of dietary treatment on the duodenal crypt depth measurement (P = 0.03). Supplementation of B. subtilis DSM 32315 significantly decreased the crypt depth compared to the IC treatment, and was significantly different from the BMD treatment, but not significantly different from the NC treatment. There was a significant effect of dietary treatment on the midintestinal crypt depth (P = 0.04) and villus to crypt depth (VCD) ratio (P = 0.02). The mid-intestine crypt depth was significantly increased, while VCD was significantly decreased in IC compared to the NC. However, neither B. subtilis DSM 32315 nor BMD treatment was significantly different from IC for both mid-intestine crypt depth and VCD. The cecal mucosal thickness was significantly increased in the NC group compared to the IC group (P = 0.04). However, neither B. subtilis DSM 32315 nor BMD supplementation signifbl2icantly increased the cecal mucosal thickness of birds, in comparison to the IC group.
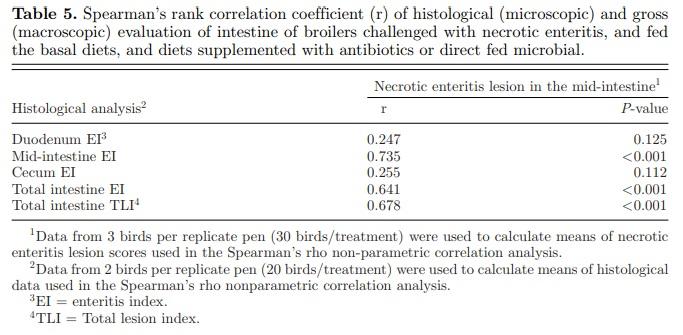
Although, as expected, coccidia stages of E. maxima was observed in both the duodenum and mid-intestine, there was no significant treatment effect on mean CI in both intestinal segments. However, there were significant dietary effects on duodenal TLI (P = 0.05), mid-intestinal EI (P <0.01) and TLI (P <0.01), cecal EI (P = 0.04), and total intestine EI (P <0.01) and TLI (P <0.01). The duodenal and mid-intestinal TLI were reduced in the NC treatment compared to the IC treatment. However, supplementation of B. subtilis DSM 32315 and BMD only tended to reduce the duodenal TLI. The supplementation of B. subtilis DSM 32315 significantly reduced the mid-intestinal TLI and EI compared to the IC treatment. In the cecum, EI was significantly reduced with B. subtilis DSM 32315 supplementation, in comparison to the IC treatment, and was not significantly different from the NC treatment. Furthermore, the cecal EI was significantly lower in the B. subtilis DSM 32315 treatment group compared to the BMD treatment group. Total intestine EI and TLI were significantly lower in the B. subtilis DSM 32315 treatment compared to the IC treatment, and this response was not significantly different from the BMD treatment. The results of correlation analysis between the intestinal macroscopic and microscopic pathology are reported in Table 5. A positive statistical correlation was observed between the mid-intestinal EI and mid-intestinal NE lesion score. Furthermore, there was a positive statistical correlation between the total intestine EI and TLI, and macroscopic NE lesion score. However, there was no correlation between the duodenal and cecal EI and macroscopic NE lesion scores.
Experiment 2
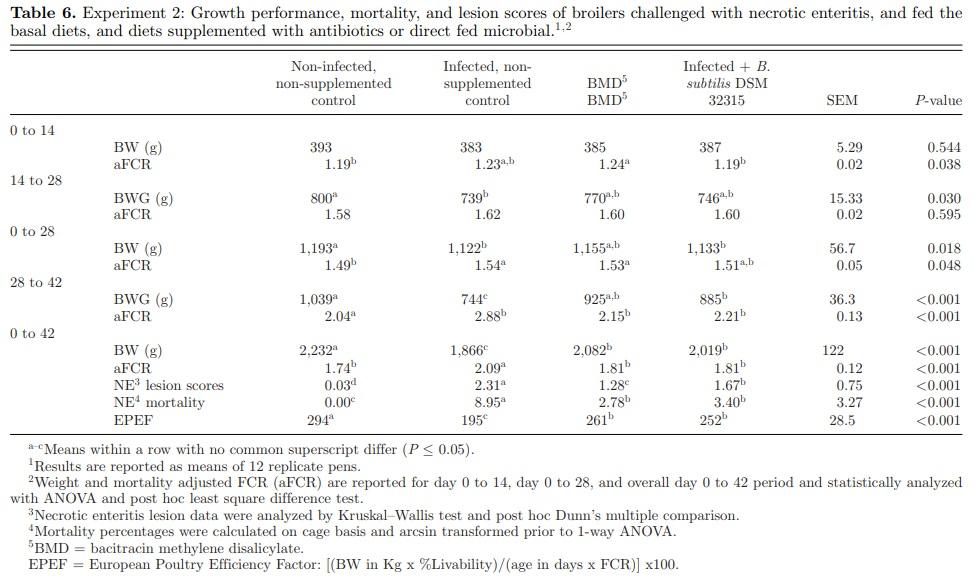
The growth performance, NE mortality, and intestinal lesion scores are presented in Table 6. At day 14, BW of birds was not significantly different (P = 0.544) across dietary treatments. However, there was a significant (P = 0.038) treatment effect on aFCR, with aFCR being significantly lower in the NC and B. subtilis DSM 32315 treatment groups, in comparison to BMD treatment. During the challenge phase (day 14 to 28), there was a significant difference in BWG between the NC and IC group, with both BMD and B. subtilis DSM 32315 being intermediate and only tended to be significantly different from the NC and IC groups. At day 28, the effect of the NE challenge was apparent. A significant difference in BW (P = 0.018) and aFCR (P = 0.048) between the NC and IC groups was observed. However, there was no statistically significant difference in BW and aFCR between the IC, BMD, and B. subtilis DSM 32315 groups. During the recovery phase of the challenge (day 28 to 42), BWG of birds in the IC and B. subtilis DSM 32315 groups was significantly lower than birds in the NC group. However, BWG of birds in the B. subtilis DSM 32315 and BMD groups was significant higher than birds in the IC group. There was no significant difference in BWG between the BMD and B. subtilis DSM 32315 groups, and between the NC and BMD groups. Furthermore, the aFCR was significantly higher in the IC group in comparison to other treatment groups, and there was no significant difference in aFCR among the other treatment groups (NC, BMD, and B. subtilis DSM 32315). At day 42, there was significant effect of dietary treatment on BW of birds (P < 0.001). The BW of birds in the IC group was significantly lower than birds in the NC group, and the supplementation of BMD and B. subtilis DSM 32315 significantly increased the BW by 216 g (9%) and 153 g (8%), respectively, compared to the IC group, with no significant difference between the BMD and B. subtilis DSM 32315 groups. Similarly, there was a significant treatment effect on aFCR (P < 0.001). The aFCR was highest in birds in the IC group compared to the NC, BMD, and B. subtilis DSM 32315 groups. The supplementation of BMD and B. subtilis DSM 32315 both improved the aFCR and neither treatment was statistically different from the NC.
There was a significant effect of dietary treatment on NE-associated mortality (P < 0.001). Mortality was significantly higher in the IC group compared to the NC group; however, supplementation of BMD or B. subtilis DSM 32315 significantly reduced the NE mortality. There was a significant treatment effect on the mean intestinal NE lesion score. Mean score was significantly higher in the IC group and lowest in the NC group. However, lesions were significantly reduced by BMD and B. subtilis DSM 32315 supplementation. There was significant treatment effect on the EPEF (P < 0.001). The EPEF was significantly lower in the IC group compared to the NC group, with the BMD and B. subtilis DSM 32315 treated groups being intermediate and not significantly different.
DISCUSSION
The primary objective of this study was to investigate the effect of dietary supplementation of B. subtilis DSM 32315 on the intestinal health and growth performance of broilers under an experimental NE challenge. Previous in vitro data (unpublished) have demonstrated the ability of B. subtilis DSM 32315 strain to inhibit C. perfringens. In addition, Whelan et al. (2018) demonstrated that supplementation of B. subtilis DSM 32315 can support the reduction of C. perfringens in the intestine, as well as the stabilization of the intestinal microbiome and improvement of growth performance, in broilers challenged with NE. It was, therefore, hypothesized in the current study that B. subtilis DSM 32315 may prevent the detrimental impact of NE on the intestinal architecture and growth performance of broiler chickens. Therefore, 2 experiments were conducted to test this hypothesis, using 2 control sources (IC and NC), and BMD (approved for the control of NE due to C. perfringens) as a standard comparison. In both experiments, the performance at the end of each cycle at either day 28 (experiment 1) or day 42 (experiment 2) showed that the in-feed supplementation of B. subtilis DSM 32315 beginning at the starter phase was effective in reducing the effect of NE and improving growth performance in a manner comparable to BMD. In addition, in experiment 2, a positive effect of B. subtilis DSM 32315 on performance was observed during the NE challenge phase (day 14 to 28), and the effect was more evident in the post-challenge phase (day 28 to 42). The quantifiable improvement suggests that B. subtilis DSM 32315 can be used as a non-antibiotic feed additive to control NE resulting in growth performance that is comparable to the use BMD. Several studies have shown through different mechanisms, the natural ability of specific strains of B. subtilis to improve NE-associated pathology and performance of broiler chickens (Teo and Tan, 2005; Jayaramna et al., 2013; Tactacan et al., 2013; Krueger et al., 2017; Latorre et al., 2017; Rhayat et al., 2017; Bortoluzzi et al., 2019). The microscopic pathology associated with NE includes a severe inflammatory response characterized by presence of immune cell populations (heterophils, lymphocytes, macrophages, and plasma cells), and damage to the villi, crypt, and intestinal submucosa (Opengart, 2008). In the current study, it was demonstrated that dietary supplementation of B. subtitlis DSM 32315 influenced the small intestine crypt depth and VCD ratio.
This suggests that B. subtilis DSM 32315 supplementation lowered the rate of epithelial cell proliferation and tissue turnover at the crypt region. This finding agrees with Ma et al. (2018) in which B. subtilis DSM 32315 supplementation significantly improved the villus height and VCD of ileum compared to the control group. Similarly, Latorre et al. (2017) and Jayaraman et al. (2013) both reported a significant increase in villus height and VCD, and a reduction in crypt depth in birds fed 2 different Bacillus DFM strains. Villi height, crypt depth, and their ratio can be important indicator of intestinal integrity and functionality. Long villi and shallow crypts are indicative of optimal intestinal integrity and absorptive capacity which can support growth performance (Wu et al., 2004; Hu et al., 2013). In addition to the benefits on intestinal morphology, B. subtilis DSM 32315 also attenuated intestinal inflammation (enteritis index) measured by histopathology.
The negative impact of immune system stimulation on digestion and growth performance has been demonstrated in several studies. Rochell et al. (2016) showed that a dose-dependent infection with sporulated oocysts of E. acervulina negatively impacted apparent ileal digestibility of amino acids and amino acid metabolism. Additionally, Iseri and Klasing (2013) showed a higher quantifiable cost due to inflammation in acute phase response than the subsequent adaptive response. Furthermore, this response to inflammation or immune activation resulted in a significant impact on feed intake (Remus et al., 2014), BWG (Rochell et al., 2016), and increased nutritional requirement (Klasing, 2007; Rochell et al., 2016). The modulation of enteric inflammation by probiotics is thought to result from intestinal microbiota management rather than by inhibition of inflammatory cell infiltration or mediator production (Tellez et al., 2006; Niewold, 2007; Whelan et al., 2018). The reduction of inflammation may have positive effect on growth performance by preventing the repartitioning of nutrients away from growth, and mobilization of immune cells which requires energy dispensation. Therefore, in the current study, B. subtilis DSM 32315 supported the reduction of enteritis and improvement of the intestinal architecture, likely by inhibiting C. perfringens, and supporting the intestinal microflora balance (Ma et al., 2018; Whelan et al., 2018; Bortoluzzi et al., 2019), resulting in improved growth performance.
Although supplementation of B. subtilis DSM 32315 did not improve cecal mucosa thickness, it did reduce cecal enteritis. This may be supported by previous reports that B. subtilis DSM 32315 supplementation tended to affect cecal microbiota metabolic pathways under non-challenge conditions (Ma et al., 2018), and significantly affected population changes in the cecal microbiota (Whelan et al., 2018; Bortoluzzi et al., 2019) and the predicted functions of the cecal digesta microbiota (Bortoluzzi et al., 2019) under NE challenge conditions. This suggests that B. subtilis DSM 32315 may support ceca health by modulating the ceca microbiota, thereby reducing cecal inflammation of NE-infected chickens. Under this NE infection model, we showed that histological evaluation of NE-associated enteritis was significantly correlated with lesion score (gross pathology). This may indicate that lesion scoring, which is widely used in the field for the diagnosis of NE, continues to have relevance (Opengart, 2008; Teirlynck et al., 2011). Furthermore, a stronger association observed in the mid-intestine in comparison to other segments may be related to the fact that NE lesions occur primarily in the mid-intestine.
CONCLUSIONS
NE challenge impaired the performance of broiler chickens. However, dietary supplementation with B. subtilis DSM 32315 attenuated this effect evident by the reduction of NE lesions and mortality, and improvement in growth performance. Additionally, B. subtilis DSM 32315 supplementation reduced enteritis and positively affected intestinal morphology, which may be a partial mechanism by which it improved performance of broiler chickens in the NE challenge. Future research should investigate the effects of B. subtilis DSM 32315 on the modulation of the immune system in response to inflammation associated with NE challenge.
ACKNOWLEDGMENTS
The authors would like to acknowledge Dr. Fred Hoerr and Dr. J. Schrader of Veterinary Diagnostic Pathology, LLC for providing the expertise on all the histopathological analysis. Authors’ Contributions: AOS, AM, and KD were responsible for the design of the experiments. GFM, BL, and MDS conducted the experiments and collected the samples. AOS was responsible for writing the manuscript. AOS, RAW, AM, and KD were responsible for editing the manuscript. All authors read and approved the final version of the manuscript.
Conflict of interest statement
The authors declare that there are no conflicts of interest.