Managing Free-Range Laying Hens—Part B: Early Range Users Have More Pathology Findings at the End of Lay but Have a Significantly Higher Chance of Survival—An Indicative Study
Free-range facilities may present a biosecurity risk in some situations, but range use has also been associated with better hen welfare. We investigated the association between early-life range use (when hens were 18–21 weeks of age) and hen survival during the entire housing period as well as various health and welfare parameters at 74 weeks of age. Hens that preferred to use the range at early life were three times more likely to survive. Early range users were also 1.6 times more likely to become infected with gastrointestinal nematodes and showed significantly more frequent signs indicating spotty liver disease. Hens that preferred to stay in the shed during early life had a higher prevalence of Fatty Liver Syndrome and significantly less feather cover. In conclusion, hens that do not range during early life may benefit from additional management strategies to increase their likelihood of survival. Further investigations under controlled environmental conditions are warranted to quantify further the observed effects.
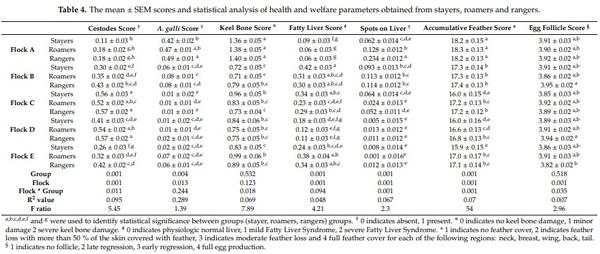
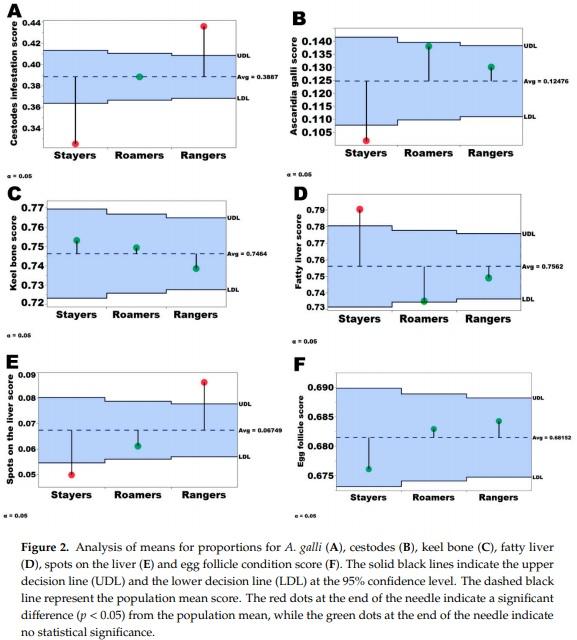
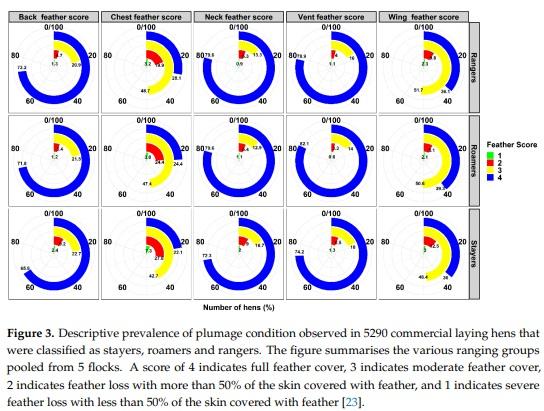
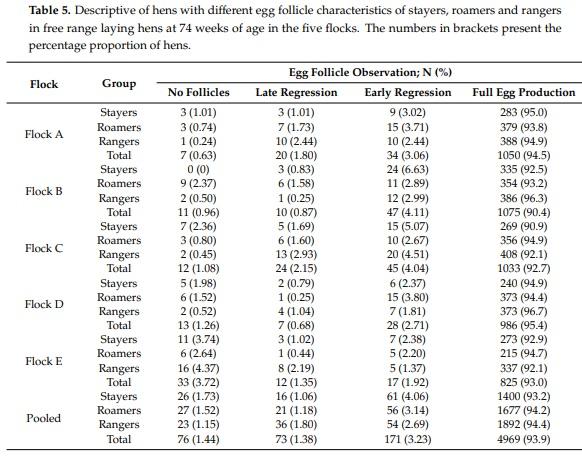
Abstract: While free-range laying hens frequently experience health and welfare challenges, the contribution of range use towards these risks are largely unknown. The aim of this pilot study was to investigate the survival, health and welfare of commercial free-range laying hens and explore the association with early range use. Range use of 9375 Lohmann Brown hens housed within five flocks was assessed during 18–21 weeks of age and individual hens were classified as “rangers” (frequent range users), “roamers” (intermittent range users), and “stayers” (rare/no range users) were then subject to necropsy at 74 weeks of age. Rangers and roamers were three times and 2.4 times more likely to survive than stayers, respectively (p = 0.001). Overall, rangers had significantly better feather cover and more lesions associated with spotty liver diseases compared to roamers and stayers (p = 0.001). Similarly, rangers and roamers had a higher prevalence of A. galli infection and less frequent signs of fatty liver syndrome compared to stayers. Rangers had a higher proportion of hens with full ovary follicle production compared to stayers and roamers (p = 0.035). This information is highly relevant to consider the targeted support of different flock subpopulations to improve hen health and welfare, directly affecting farm profitability. Further research on other farms is warranted to investigate the transferability of the observed results.
Keywords: behaviour; egg; feather cover; health; housing; mortality; non-caged; parasites; poultry; roundworms; tapeworms; welfare.
1. Rodenburg, T.B.; Tuyttens, F.A.M.; de Reu, K.; Herman, L.; Zoons, J.; Sonck, B. Welfare assessment of laying
hens in furnished cages and non-cage systems: An on-farm comparison. Anim. Welf. 2008, 17, 363–373.
2. Lay, D.C.; Fulton, R.M.; Hester, P.Y.; Karcher, D.M.; Kjaer, J.B.; Mench, J.A.; Mullens, B.A.; Newberry, R.C.;
Nicol, C.J.; O’Sullivan, N.P.; et al. Hen welfare in different housing systems. Poult. Sci. 2011, 90, 278–294.
[CrossRef] [PubMed]
3. Freire, R.; Cowling, A. The welfare of laying hens in conventional cages and alternative systems: First steps
towards a quantitative comparison. Anim. Welf. 2013, 22, 57–65. [CrossRef]
4. Sherwin, C.; Nasr, M.; Gale, E.; Petek, M.; Stafford, K.; Turp, M.; Coles, G.C. Prevalence of nematode infection
and faecal egg counts in free-range laying hens: Relations to housing and husbandry. Br. Poult. Sci. 2013,
54, 12–23. [CrossRef] [PubMed]
5. De Wit, J.J.; Koch, G.; Fabri, T.H.F.; Elbers, A.R.W. A cross-sectional serological survey of the Dutch commercial
poultry population for the presence of low pathogenic avian influenza virus infections. Avian Pathol. 2004,
33, 565–570. [CrossRef]
6. Elbers, A.R.W.; Fabri, T.H.F.; De Vries, T.S.; De Wit, J.J.; Pijpers, A.; Koch, G. The Highly Pathogenic Avian
Influenza A (H7N7) Virus Epidemic in the Netherlands in 2003—Lessons Learned from the First Five
Outbreaks. Avian Dis. 2004, 48, 691–705. [CrossRef]
7. Couto, R.M.; Braga, J.F.; Gomes, S.Y.M.; Resende, M.; Martins, N.R.; Ecco, R. Natural concurrent infections
associated with infectious laryngotracheitis in layer chickens. J. Appl. Poult. Res. 2016, 25, 113–128. [CrossRef]
8. Dewulf, J.; Van Hoorebeke, S.; Van Immerseel, F. Epidemiology of Salmonella infections in laying hens with
special emphasis on the influence of the housing system. In Improving the Safety and Quality of Eggs and Egg
Products; Elsevier BV: Amsterdam, The Netherlands, 2011; pp. 107–119.
9. Zhang, P.; Fegan, N.; Fraser, I.; Duffy, P.; Bowles, R.E.; Gordon, A.; Ketterer, P.J.; Shinwari, W.; Blackall, P.J.
Molecular epidemiology of two fowl cholera outbreaks on a free-range chicken layer farm. J. Vet.
Diagn. Investig. 2004, 16, 458–460. [CrossRef]
10. Alhaji, N.B.; Odetokun, I.A. Assessment of Biosecurity Measures Against Highly Pathogenic Avian
Influenza Risks in Small-Scale Commercial Farms and Free-Range Poultry Flocks in the Northcentral
Nigeria. Transbound. Emerg. Dis. 2011, 58, 157–161. [CrossRef]
11. Park, H.-S.; Min, B.; Oh, S.-H. Research trends in outdoor pig production—A review. Asian-Australas. J.
Anim. Sci. 2017, 30, 1207–1214. [CrossRef]
12. Li, Q. Effect of forest bathing trips on human immune function. Environ. Health Prev. Med. 2009, 15, 9–17.
[CrossRef] [PubMed]
13. Riihimäki, M.; Raine, A.; Elfman, L.; Pringle, J. Markers of respiratory inflammation in horses in relation to
seasonal changes in air quality in a conventional racing stable. Can. J. Vet. Res. 2008, 72, 432–439. [PubMed]
14. Rodenburg, T.B.; Van Krimpen, M.M.; De Jong, I.C.; De Haas, E.N.; Kops, M.S.; Riedstra, B.J.; Nordquist, R.E.;
Wagenaar, J.P.; Bestman, M.; Nicol, C.J. The prevention and control of feather pecking in laying hens:
Identifying the underlying principles. World’s Poult. Sci. J. 2013, 69, 361–374. [CrossRef]
15. Cronin, G.; Hopcroft, R.; Groves, P.; Hall, E.; Phalen, D.; Hemsworth, P. Why did severe feather pecking and
cannibalism outbreaks occur? An unintended case study while investigating the effects of forage and stress
on pullets during rearing. Poult. Sci. 2018, 97, 1484–1502. [CrossRef] [PubMed]
16. Bestman, M.; Wagenaar, J. Farm level factors associated with feather pecking in organic laying hens.
Livest. Prod. Sci. 2003, 80, 133–140. [CrossRef]
17. Drake, K.; Donnelly, C.A.; Dawkins, M.S. Influence of rearing and lay risk factors on propensity for feather
damage in laying hens. Br. Poult. Sci. 2010, 51, 725–733. [CrossRef]
18. Lambton, S.L.; Knowles, T.G.; Yorke, C.; Nicol, C.J. The risk factors affecting the development of gentle and
severe feather pecking in loose housed laying hens. Appl. Anim. Behav. Sci. 2010, 123, 32–42. [CrossRef]
19. Lambton, S.L.; Nicol, C.J.; Friel, M.; Main, D.C.J.; McKinstry, J.L.; Sherwin, C.M.; Walton, J.; Weeks, C.A.
A bespoke management package can reduce levels of injurious pecking in loose-housed laying hen flocks.
Vet. Rec. 2013, 172, 423. [CrossRef]
20. Campbell, D.L.M.; Hinch, G.N.; Dyall, T.R.; Warin, L.; Little, B.A.; Lee, C. Outdoor stocking density in
free-range laying hens: Radio-frequency identification of impacts on range use. Animal 2016, 11, 121–130.
[CrossRef]
21. Blokhuis, H.; Wiepkema, P. Studies of feather pecking in poultry. Vet. Q. 1998, 20, 6–9. [CrossRef]
22. Huber-Eicher, B.; Audige, L. Analysis of risk factors for the occurrence of feather pecking in laying hen
growers. Br. Poult. Sci. 1999, 40, 599–604. [CrossRef]
23. Tauson, R.; Kjaer, J.B.; Levrino, G.A.; Cepero Briz, R. Applied scoring of integument and health in laying
hens. Pol. Acad. Sci. 2005, 23 (Suppl. 1), 153–159.
24. Wilkins, L.J.; McKinstry, J.L.; Avery, N.C.; Knowles, T.G.; Brown, S.N.; Tarlton, J.; Nicol, C.J. Influence of
housing system and design on bone strength and keel bone fractures in laying hens. Vet. Rec. 2011, 169, 414.
[CrossRef] [PubMed]
25. Gebhardt-Henrich, S.G.; Toscano, M.; Fröhlich, E.K. Use of outdoor ranges by laying hens in different sized
flocks. Appl. Anim. Behav. Sci. 2014, 155, 74–81. [CrossRef]
26. Gilani, A.-M.; Knowles, T.G.; Nicol, C.J. Factors affecting ranging behaviour in young and adult laying hens.
Br. Poult. Sci. 2014, 55, 127–135. [CrossRef] [PubMed]
27. Sibanda, T.Z.; Walkden-Brown, S.W.; Kolakshyapati, M.; Dawson, B.; Schneider, D.; Welch, M.; Iqbal, Z.;
Cohen-Barnhouse, A.; Morgan, N.K.; Boshoff, J.; et al. Flock use of the range is associated with the use of
different components of a multi-tier aviary system in commercial free-range laying hens. Br. Poult. Sci. 2019,
61, 97–106. [CrossRef] [PubMed]
28. Sibanda, T.Z.; Kolakshyapati, M.; Welch, M.; Schneider, D.; Boshoff, J.; Ruhnke, I. Managing Free-Range
Laying Hens—Part A: Frequent and Non-Frequent Range Users Differ in Laying Performance but Not Egg
Quality. Animals 2020, 10, 991. [CrossRef]
29. Sibanda, T.Z.; Dawson, B.; Welch, M.; Schneider, D.; Boshoff, J.; Kolakshyapati, M.; Ruhnke, I. Validation of
a Radio Frequency Identification (RFID) system for aviary systems. In Proceedings of the 31th Australian
Poultry Science Symposium, Sydney, Australia, 16–19 February 2020.
30. Tauson, R. Furnished cages and aviaries: Production and health. World’s Poult. Sci. J. 2002, 58, 49–63.
[CrossRef]
31. Zuur, A.F.; Ieno, E.N. A protocol for conducting and presenting results of regression-type analyses.
Methods Ecol. Evol. 2016, 7, 636–645. [CrossRef]
32. Zuur, A.F.; Ieno, E.N.; Walker, N.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology
with R; Springer: New York, NY, USA, 2009.
33. Humphrey, T. Are happy chickens safer chickens? Poultry welfare and disease susceptibility. Br. Poult. Sci.
2006, 47, 379–391. [CrossRef]
34. Kaufmann, F.; Da¸s, G.; Sohnrey, B.; Gauly, M. Helminth infections in laying hens kept in organic free range
systems in Germany. Livest. Sci. 2011, 141, 182–187. [CrossRef]
35. Thapa, S.; Hinrichsen, L.K.; Brenninkmeyer, C.; Gunnarsson, S.; Heerkens, J.L.; Verwer, C.; Niebuhr, K.;
Willett, A.; Grilli, G.; Thamsborg, S.M.; et al. Prevalence and magnitude of helminth infections in organic
laying hens (Gallus gallus domesticus) across Europe. Vet. Parasitol. 2015, 214, 118–124. [CrossRef] [PubMed]
36. Sharma, N.; Hunt, P.W.; Hine, B.C.; Ruhnke, I. The impacts of Ascaridia galli on performance, health,
and immune responses of laying hens: New insights into an old problem. Poult. Sci. 2019, 98, 6517–6526.
[CrossRef] [PubMed]
37. Da Silva, G.S.; Romera, D.M.; Conhalato, G.D.S.; Soares, V.E.; Meireles, M.V. Helminth infections in chickens
(Gallus domesticus) raised in different production systems in Brazil. Vet. Parasitol. Reg. Stud. Rep. 2018,
12, 55–60. [CrossRef] [PubMed]
38. Crawshaw, T.; Young, S. Increased mortality on a free-range layer site. Vet. Rec. 2003, 153, 664.
39. Grimes, T.; Reece, R. Spotty liver disease—An emerging disease in free range egg layers in Australia.
In Proceedings of the 16th Western Poultry Disease Conference, Sacramento, CA, USA, 20–23 March 2011;
pp. 53–56.
40. Courtice, J.M.; Mahdi, L.K.; Groves, P.J.; Kotiw, M. Spotty Liver Disease: A review of an ongoing challenge
in commercial free-range egg production. Vet. Microbiol. 2018, 227, 112–118. [CrossRef]
41. Godoy, P.; Hewitt, N.J.; Albrecht, U.; Andersen, M.E.; Ansari, N.; Bhattacharya, S.; Bode, J.G.; Bolleyn, J.;
Borner, C.; Böttger, J.; et al. Recent advances in 2D and 3D in vitro systems using primary hepatocytes,
alternative hepatocyte sources and non-parenchymal liver cells and their use in investigating mechanisms of
hepatotoxicity, cell signaling and ADME. Arch. Toxicol. 2013, 87, 1315–1530. [CrossRef] [PubMed]
42. Malarkey, D.E.; Johnson, K.; Ryan, L.; Boorman, G.; Maronpot, R.R. New Insights into Functional Aspects of
Liver Morphology. Toxicol. Pathol. 2005, 33, 27–34. [CrossRef]
43. Shini, A.; Shini, S.; Bryden, W.L. Fatty liver haemorrhagic syndrome occurrence in laying hens: Impact of
production system. Avian Pathol. 2018, 48, 25–34. [CrossRef]
44. Squires, E.; Leeson, S. Aetiology of fatty liver syndrome in laying hens. Br. Vet. J. 1988, 144, 602–609.
[CrossRef]
45. Griffith, M.; Olinde, A.J.; Schexnailder, R.; Davenport, R.F.; McKnight, W.F. Effect of Choline, Methionine
and Vitamin B12 on Liver Fat, Egg Production and Egg Weight in Hens. Poult. Sci. 1969, 48, 2160–2172.
[CrossRef]
46. Grobas, S.; Mendez, J.; De Blas, J.C.; Mateos, G.G. Laying hen productivity as affected by energy, supplemental
fat, and linoleic acid concentration of the diet. Poult. Sci. 1999, 78, 1542–1551. [CrossRef]
47. Mika, A.; Macaluso, F.; Barone, R.; Di Felice, V.; Sledzinski, T. Effect of Exercise on Fatty Acid Metabolism
and Adipokine Secretion in Adipose Tissue. Front. Physiol. 2019, 10, 26. [CrossRef] [PubMed]
48. Watt, M.J.; Heigenhauser, G.J.F.; Dyck, D.J.; Spriet, L.L. Intramuscular triacylglycerol, glycogen and acetyl
group metabolism during 4 h of moderate exercise in man. J. Physiol. 2002, 541, 969–978. [CrossRef]
49. Chielo, L.I.; Pike, T.W.; Cooper, J.J. Ranging Behaviour of Commercial Free-Range Laying Hens. Animals
2016, 6, 28. [CrossRef] [PubMed]
50. Mahboub, H.D.H.; Müller, J.; Von Borell, E. Outdoor use, tonic immobility, heterophil/lymphocyte ratio and
feather condition in free-range laying hens of different genotype. Br. Poult. Sci. 2004, 45, 738–744. [CrossRef]
[PubMed]
51. Hartcher, K.M.; Hickey, K.A.; Hemsworth, P.H.; Cronin, G.M.; Wilkinson, S.J.; Singh, M. Relationships
between range access as monitored by radio frequency identification technology, fearfulness, and plumage
damage in free-range laying hens. Animals 2015, 10, 847–853. [CrossRef]
52. Tauson, R.; Ambrosen, T.; Elwinger, K. Evaluation of Procedures for Scoring the Integument of Laying
Hens—Independent Scoring of Plumage Condition. Acta Agric. Scand. 1984, 34, 400–408. [CrossRef]
53. Kjaer, J. Diurnal rhythm of feather pecking behaviour and condition of integument in four strains of loose
housed laying hens. Appl. Anim. Behav. Sci. 2000, 65, 331–347. [CrossRef]
54. Yamak, U.; Sarica, M. Relationships between feather score and egg production and feed consumption of
different layer hybrids kept in conventional cages. Archiv. Geflugelkd. 2012, 76, 31–37.
55. Sherwin, C.; Richards, G.; Nicol, C. Comparison of the welfare of layer hens in 4 housing systems in the UK.
Br. Poult. Sci. 2010, 51, 488–499. [CrossRef] [PubMed]
56. Käppeli, S.; Gebhardt-Henrich, S.; Fröhlich, E.; Pfulg, A.; Stoffel, M.H. Prevalence of keel bone deformities in
Swiss laying hens. Br. Poult. Sci. 2011, 52, 531–536. [CrossRef] [PubMed]
57. Tarlton, J.; Wilkins, L.J.; Toscano, M.J.; Avery, N.C.; Knott, L. Reduced bone breakage and increased bone
strength in free range laying hens fed omega-3 polyunsaturated fatty acid supplemented diets. Bone 2013,
52, 578–586. [CrossRef] [PubMed]
58. Petrik, M.T.; Guerin, M.T.; Widowski, T.M. On-farm comparison of keel fracture prevalence and other
welfare indicators in conventional cage and floor-housed laying hens in Ontario, Canada. Poult. Sci. 2015,
94, 579–585. [CrossRef]
59. Stratmann, A.; Fröhlich, E.K.F.; Harlander-Matauschek, A.; Schrader, L.; Toscano, M.J.; Würbel, H.;
Gebhardt-Henrich, S.G. Soft Perches in an Aviary System Reduce Incidence of Keel Bone Damage in
Laying Hens. PLoS ONE 2015, 10, e0122568. [CrossRef]
60. Rufener, C.; Abreu, Y.; Asher, L.; Berezowski, J.A.; Sousa, F.M.; Stratmann, A.; Toscano, M.J. Keel bone
fractures are associated with individual mobility of laying hens in an aviary system. Appl. Anim. Behav. Sci.
2019, 217, 48–56. [CrossRef]
61. Casey-Trott, T.M.; Korver, D.R.; Guerin, M.T.; Sandilands, V.; Torrey, S.; Widowski, T.M. Opportunities for
exercise during pullet rearing, Part I: Effect on the musculoskeletal characteristics of pullets. Poult. Sci. 2017,
96, 2509–2517. [CrossRef]
62. Toscano, M.; Dunn, I.C.; Christensen, J.-P.; Petow, S.; Kittelsen, K.; Ulrich, R. Explanations for keel bone
fractures in laying hens: Are there explanations in addition to elevated egg production? Poult. Sci. 2020,
99, 4183–4194. [CrossRef









.jpg&w=3840&q=75)