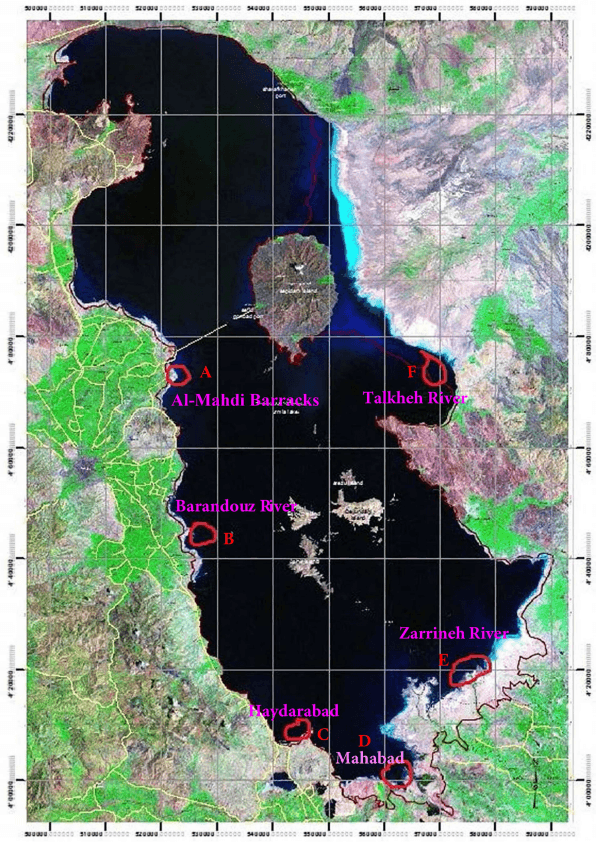
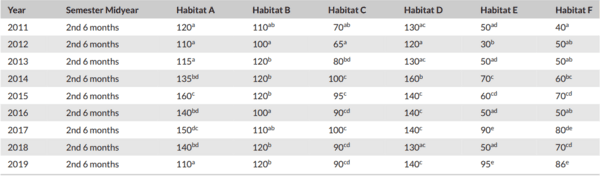
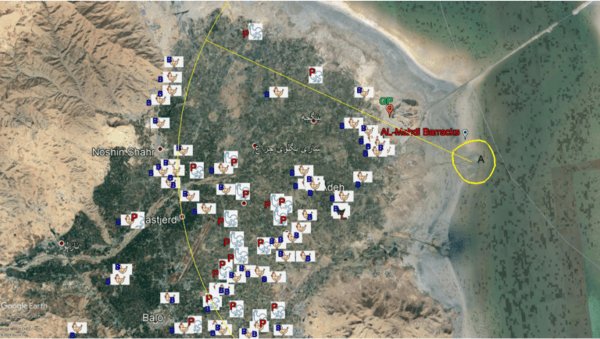
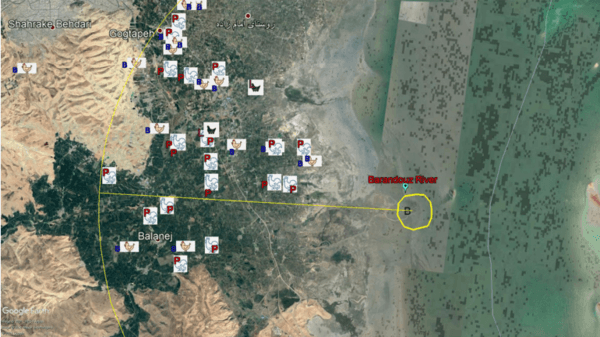
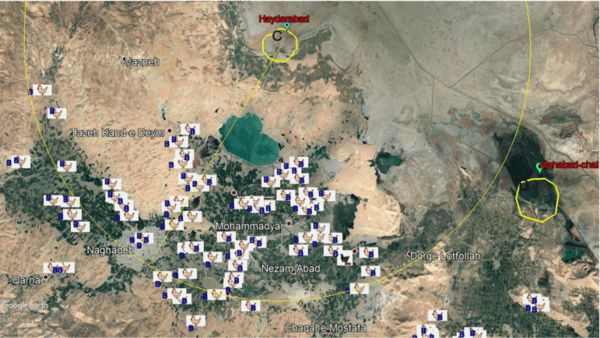
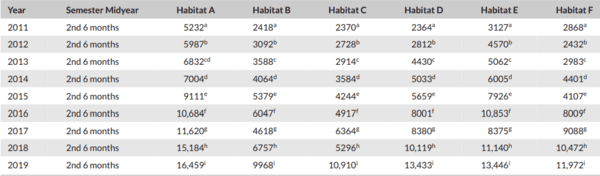
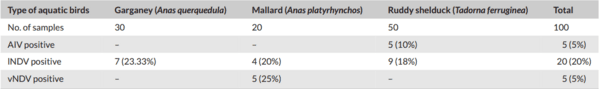
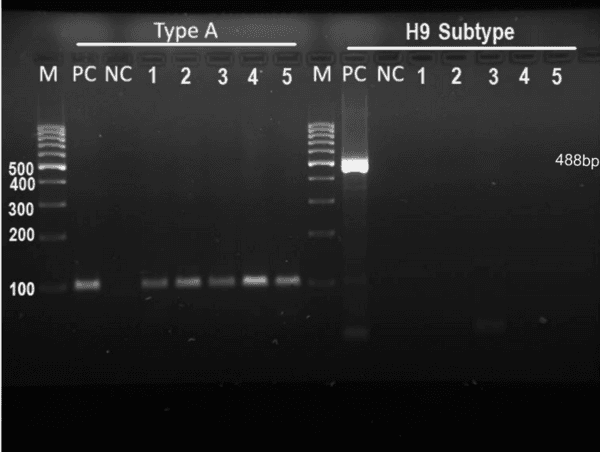
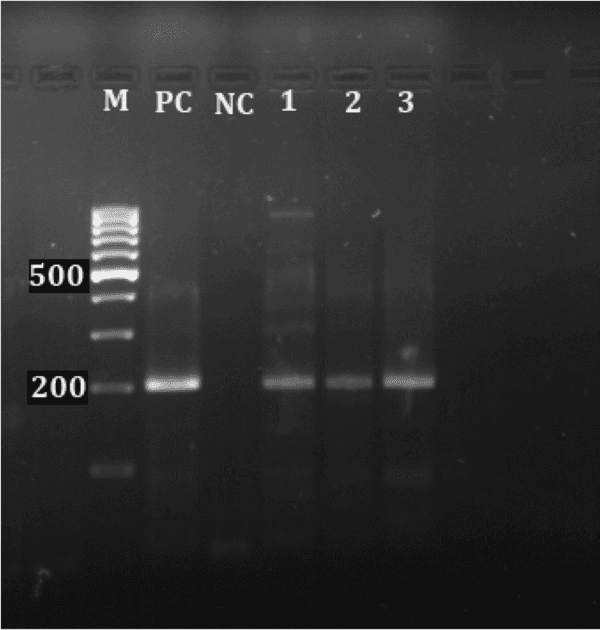
Monitoring of aquatic birds and surveillance of avian influenza and Newcastle disease of waterfowls at the National Park of Urmia Lake

Abd-Elfatah, K. S., Elabasy, M. A., El-khyate, F., Elmahallawy, E. K., Mosad,
S. M., El-Gohary, F. A., Abdo, W., Al-Brakati, A., Seadawy, M. G., Tahoon,
A. E., & El-Gohary, A. E. (2021). Molecular characterization of velogenic
Newcastle disease virus (sub-genotype VII.1.1) from wild birds, with assessment of its pathogenicity in susceptible chickens. Animals (Basel),
11(2), 505. https://doi.org/10.3390/ani11020505
Abdollahi, H., Maken Ali, A. S., Monne, I., Milani, A., Habibi, M., Zamperin,
G., Ghafouri, S. A., Maghsoudloo, H., Mohammadpoor, B., Esmaeilzadeh,
S., Farahani, R. K., Ghasemi, Y., Afzalkhani, A. A., Nabipoor, J., Javanmardi,
B., Fusaro, A., & Zecchin, B. (2020). Spatial spread and emergence of reassortant H5 highly pathogenic avian influenza viruses in Iran reassortant
H5 highly pathogenic avian influenza virus in Iran. Infection, Genetics and Evolution, 83, 104342. https://doi.org/10.1016/j.meegid.2020.
104342
Akpinar, E., & Saatci, E. (2006). Avian influenza in Turkey– Will it influence health in all Europe? Croatian Medical Journal, 47(1), 7–15.
Al-Badry, M., & Al-Mubarak, F. (2020). Molecular surveillance of avian influenza A viruses in Basrah and Wasit, Iraq. Bulgarian Journal of Veterinary Medicine, 23(4), 456–466. https://doi.org/10.15547/bjvm.2019-
0016
Al-Shekaili, T., Clough, H., Ganapathy, K., & Baylis, M. (2015). Serosurveillance and risk factors for avian influenza and Newcastle disease virus in backyard poultry in Oman. Preventive Veterinary Medicine, 12(1–
2), 145–153. https://doi.org/10.1016/j.prevetmed.2015.09.011
Ashouri, A., Vasfi-Marandi, M., Ghalyanchi-Langeroudi, A., Karimi, V., Ziafati,
Z., & Hosseini, H. (2019). Genetic analysis of avian Orthoavulavirus type I (AOAV-1) strains isolated from broiler flocks. Iranian Journal of Virology, 13(2), 16–23. https://www.sid.ir/en/journal/ViewPaper.aspx? id=797466
Anonymous. (2021). Wetlands International. “Water bird Population Estimates.” Retrieved from wpe.wetlands.org (http://wpe.wetlands.org) on
Sunday 15 Aug 2021
Bashashati, M., Vasfi-Marandi, M., & Sabouri, F. (2013). Genetic diversity of early (1998) and recent (2010) avian influenza H9N2 virus strains isolated from poultry in Iran. Archives of Virology, 158(10), 2089–2100. https://doi.org/10.1007/s00705-013-1699-2
Bergervoet, S. A., Germeraad, E. A., Alders, M., Roose, M. M., Engelsma, M. Y.,
Heutink, R., Bouwstra, R., Fouchier, R. A. M., & Beerens, N. (2019). Susceptibility of chickens to low pathogenic avian influenza (LPAI) Viruses of wild bird– and poultry–associated subtypes. Viruses, 11(11), 1010. https://doi.org/10.3390/v11111010
Boynukara, B., Gülhan, T., Çöven, F., Kiziroglu, I., & Durmu¸ ˘ s, A. (2013). Determination of Newcastle disease virus among wild bird populations in Lake
Van basin, Turkey. Turkish Journal of Veterinary and Animal Sciences, 37(1),
86–93. https://doi.org/10.3906/vet-1111-26
Camenisch, G., Bandli, R., & Hoop, R. (2008). Monitoring of wild birds for
Newcastle disease virus in Switzerland using teal time RT-PCR. Journal of
Wildlife Diseases, 44(3), 772–776. https://doi.org/10.7589/0090-3558-
44.3.772
Chevallier, D., Dehorter, O., Brossard, Ch., & Larvol, J. P. (2016). ‘Teleanaesthesia’: A new approach to wild bird capture under field conditions.
Ringing & Migration, 31(1), 77–80. http://doi.org/10.1080/03078698.
2016.1190549
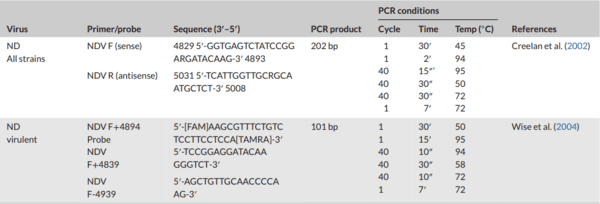
Creelan, J. L., Graham, D. A., & McCullough, S. J. (2002). Detection and differentiation of pathogenicity of avian paramyxovirus serotype
1 from field cases using one-step reverse transcriptase-polymerase chain reaction. Avian Pathology, 31(5), 493–499. https://doi.org/10.
1080/0307945021000005860
Dehghanipour, A. H., Moshir Panahi, D., Mousavi, H., Kalantari, Z., &
Tajrishy, M. (2020). Effects of water level decline in Lake Urmia, Iran, on local climate conditions. Water, 12(8), 2153. https://doi.org/10.3390/ w12082153
Dunn, E. H., Bart, J., Collins, B. T., Craig, B., Dale, B., Downes, C. M., Francis,
C. M.,Woodley, S., & Zorn, P. (2006). Monitoring bird populations in small geographic areas. Ottawa, Ontario, Canada: The CanadianWildlife Service. https://pubs.er.usgs.gov/publication/70030252
El-Naggar, R. F., Rohaim, M. A., Bazid, A. H., Ahmed, K. A., Hussein, H. A.,
& Munir, M. (2018). Biological characterization of wild-bird-origin avian avulavirus 1 and efficacy of currently applied vaccines against potential infection in commercial poultry. Archives of Virology, 163(10), 2743–
2755. https://doi.org/10.1007/s00705-018-3916-5 Epub 2018 Jun 19.
PMID: 29922856
Esmaelizadeh, M., Ashtiani, M. P., Jelokhani-niaraki, S., & Hashemnejad, K. (2012). Identification of 23 specific nucleotide patterns in the HN gene of Newcastle disease viruses isolated from Iran. Turkish Journal of Biology, 36(2), 135–142. https://dergipark.org.tr/en/download/article-file/
121112
Ezeibe, M. C. O., Egbuji, A. N., Okoroafor, O. N., Eze, J. I., Ijabo, O., Mbuko,
I. J., Ngene, A. A., Eze, I. C., Ugonabo, A. C., Sanda, M. E., & Mbuko, I.
J. (2012). Antiviral effects of a synthetic aluminum-magnesium silicate, on avian influenza virus. Health, 4(7), 429–432. http://doi.org/10.4236/ health.2012.47067
Ezeibe, M. C. O., Ijabo, O., Uzopuo, C., Okoroafor, O. N., Eze, J. I.,
Mbuko, I. J., Sanda, M. E., Animoke, P. C., & Ngene, A. A. (2011).
Effects of aluminum–magnesium silicate on Newcastle disease virus and on recovery of infected chicks. International Journal of Biological and Chemical Sciences, 5(2), 835–839. http://www.ajol.info/index.php/ ijbcs
Fallah-Mehrabadi, M. H., Bahonar, A. R., Tehrani, F., Vasfi-Marandi, M.,
Sadrzadeh, A., & Shabani, M. (2016). Determinants and sero-prevalence of avian influenza (H5 & H7 subtypes) in industrial and backyard poultry of Iran -2014. Iranian Journal of Epidemiology, 12(1), 19–27.
Fallah-Mehrabadi, M., Tehrani, F., Bahonar, A., Shoushtari, A., &
Ghalyanchilangeroudi, A. (2019). Risk assessment of the introduction and spread of highly pathogenic avian influenza viruses (H5-Subtypes) via migratory birds in Iran. Iranian Journal of Epidemiology, 14(4),
384–394. URL: http://irje.tums.ac.ir/article-1-6202-en.html
Fallah-Mehrabadi, M., Ghalyanchilangeroudi, A., Ghafouri, S., Hosseini, H.,
Zabihi Petroudi, M., Modiri Hamadan, A., Rezaee, H., Motamed Chaboki,
P., Vatandour, S., & Shayeganmehr, A. (2020). Comparison of autogenous and commercial H9N2 avian influenza vaccines in a challenge with recent dominant virus. Iranian Journal of Veterinary Research, 21(2),
109–114. https://doi.org/10.22099/ijvr.2019.33162.4937
FAO. (2007). Wild birds and avian influenza: An introduction to applied field research and disease sampling techniques. In: D. Whitworth, S. H. Newman, T. Mundkur, & P. Harris (Eds.), FAO animal production and health manual (vol. 5). Rome, Italy: FAO Animal Production and health Manual (also available at www.fao.org/avianflu)
Fereidouni, S. R., Werner, O., Starick, E., Beer, M., Harder, T. C.,
Aghakhan, M., Modirrousta, H., Amini, H., Kharrazian Moghaddam,
M., Bozorghmehrifard, M. H., Akhavizadegan, M. A., Gaidet, N., Newman,
S., Hammoumi, S., Cattoli, G., Globig, A., Hoffmann, B., Sehati, M. E.,
Masoodi, S., . . . Mettenleiter, T. C. (2010). Avian influenza virus monitoring in wintering waterbirds in Iran, 2003–2007. Virology Journal, 7, article 43 14 pages. http://www.virologyj.com/content/7/1/43
Germeraad, E. A., Sanders, P., Haggenaars, T. J., de Jong, M. C. M., Beerens,
N., & Gonzales, J. L. (2019). Virus shedding of avian influenza in poultry.
Viruses, 11(9), E812. https://doi.org/10.3390/v11090812
Habib, M., Yaqub, T., Nazir, J., Shehzad, W., Rahman, A.-U., Sohail, T.,
Mukhtar, N., Mehboob, A., Munir, M., & Shabbir, M. Z. (2018). Genomic and biological characterization of Newcastle disease viruses isolated from migratory mallards (Anas platyrhynchos). Archives of Virology, 163(8),
2179–2188. https://doi.org/10.1007/s00705-018-3840-8
Hassan, M. M., El Zowalaty, M. E., Islam, A., Rahman, M. M., Chowdhury, M.
N. U., Nine, H. S. M. Z., Rahman, M. K., Järhult, J. D., & Hoque, M. A. (2020).
Serological evidence of avian influenza in captive wild birds in a zoo and two safari parks in Bangladesh. Veterinary Science, 7(3), 122. https://doi. org/10.3390/vetsci7030122
Hoseinpour, M., Fakheri Fard, A., & Naghilli, R. (2010). Death of Urmia
Lake, a silent disaster investigating of causes, results and solutions of
Urmia Lake drying. The 1st International Applied Geological Congress,
Department of Geology, Islamic Azad University – Mashad Branch, Iran,
26–28 April 2010. http://conference.khuisf.ac.ir/DorsaPax/userfiles/ file/pazhohesh/zamin%20mashad/127.pdf
Jeihouni, M., Toomanian, A., Alavipanah, S. K., & Hamzeh, S. (2017). Quantitative assessment of Urmia Lake water using space borne multisensory data and 3D modeling. Environmental Monitoring Assessment, 189(11),
572. https://doi.org/10.1007/s10661-017-6308-5
Khatami, S., & Berndtsson, R. (2013). Urmia Lake watershed restoration in
Iran: Short and long term perspectives. In 6th International Perspective on
Water Resources & the Environment conference (IPWE 2013), January 7-9,
Izmir, Turkey. https://lucris.lub.lu.se/ws/files/5453131/5364733.pdf
Killian, M. L. (2020). Avian influenza virus sample types, collection, and handling. In: E. Spackman (Ed.) Animal influenza virus. Methods in molecular biology (Vol. 2123). New York, USA: Humana. https://doi.org/10.1007/
978-1-0716-0346-8_9
Kim, T. J. (2018). Prevention of avian influenza virus by ultraviolet radiation and prediction of outbreak by satellite parameters. Journal of Biomedical
Science and Engineering, 11(7), Article ID:86234. https://doi.org/10.4236/ jbise.2018.117015
Kim, J. K., Negovetich, N. J., Forrest, H. L., & Webster, R. G. (2009). Ducks:
The ‘‘Trojan Horses’’ of H5N1 influenza. Influenza and Other Respiratory Viruses, 3(4), 121–128. https://doi.org/10.1111/j.1750-2659.2009.
00084.x
Khawaja, J. Z., Naeem, K., Ahmad, Z., & Ahmad, S. (2005). Surveillance of avian influenza viruses in wild birds in areas adjacent to epicenter of an outbreak in Federal Capital Territory of Pakistan. International Journal of
Poultry Science, 4(1), 39–43. https://doi.org/10.3923/ijps.2005.39.43
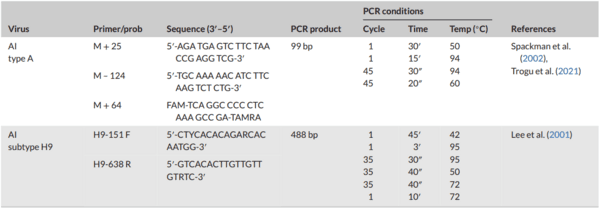
Lee, M. S., Chang, P. C., Shien, J. H, Ming-Chu Cheng, B., Happy, K., & Shieh, A. (2001). Identification and subtyping of avian influenza viruses by reverse transcription-PCR. Journal of Virology Methods, 97(1-2), 13–22. https:// doi.org/10.1016/S0166-0934(01)00301-9
Löndt, B. Z., & Alexander, D. J. (2016). Newcastle disease virus and other avian paramyxoviruses. In: S. M. Williams (Editor in Chief), Isolation, identification, and characterization of avian pathogens (6th edn., pp. 259–268).
Athens, Georgia, USA: American Association of Avian Pathologists, Inc.
Lotfi, A. (2012). Lake Uromiyeh: A concise baseline report. From the publication series of Conservation of Iranian Wetlands Project (IRI Department of Environment, United Nations Development Porgramme, Global
Environment Facilities), pp. 64–77. https://www.ulrp.ir/wp-content/ uploads/2019/04/Lake-Urmia-Baseline-Studies.pdf
Machalaba, C. C., Elwood, S. E., Forcella, S., Smith, K. M., Hamilton, K., Jebara,
K. B., Swayne, D. E., Webby, R. J., Mumford, E., Mazet, J. A., Gaidet, N.,
Daszak, P., & Karesh, W. B. (2015). Global avian influenza surveillance in wild birds: A strategy to capture viral diversity. Emerging Infectious
Diseases, 21(4), e1–e7. https://doi.org/10.3201/eid2104.141415
Madadgar, O., Karimi, V., Nazaktabar, A., Kazemimanesh, M., Ghafari, M.
M., Azimi Dezfouli, S. M., & Hojjati, P. (2013). A study of Newcastle disease virus obtained from exotic caged birds in Tehran between 2009 and
2010.Avian Pathology, 42(1), 27–31. https://doi.org/10.1080/03079457.
2012.752791
Majidzadeh, A. K., Ghalyanchi-Langeroudi, A., Soleimani, M., Karimi, V.,
& Morovat, A. (2012). Molecular surveillance of avian influenza in bird parks of Tehran, Iran. Iranian Journal of Veterinary Medicine, 6(3),
165–169. https://doi.org/10.22059/IJVM.2012.30003
Malekan, M., Vasfi-Marandi, M., Barin, A., Mokhtari-Azad, T., Ranjbar, M.,
& Bashashati, M. (2016). Molecular evaluation of M2 protein of Iranian avian influenza viruses of H9N2 subtype in order to find mutations of adamantane drug resistance. Iranian Journal of Veterinary Medicine, 10(4),
253–262. https://doi.org/10.22059/ijvm.2016.59718
Manaffar, R., Abdolahzadeh, N., Mosavi Toomatary, G., Zare, S., Sorgeloose,
P., Bossier, P., & van Stappen, G. (2020). Reproduction and life span characterization of Artemia urmiana in Lake Urmia, Iran (Branchiopoda:
Anostraca). Iranian Journal of Fisheries Sciences, 19(3), 1344–1358. https://doi.org/10.22092/ijfs.2018.116918
Miller, J. P., & Koch, G. (2020). Newcastle disease. In: D. E. Swayne (Editor in Chief), Diseases of poultry (14th edn., pp. 112—129). John
Willey & Sons, Inc. https://onlinelibrary.wiley.com/doi/book/10.1002/
9781119421481
Muzyka, D., Rula, O., Tkachenko, S., Muzyka, N., Köthe, S., Pishchanskyi, O.,
Stegniy, B., Pantin-Jackwood, M., & Beer, M. (2019). Highly pathogenic and low pathogenic avian influenza H5 subtype viruses in wild birds in Ukraine. Avian Disease, 63(sp1), 235–245. https://doi.org/10.1637/
11880-042718.1
Nasiri, F. (2020). Office of basic studies of water resources. Regional Water
Company of West-Azerbiajan (http://www.agrw.ir/). Accessed on July
10, 2020
Nhu, V. H., Mohammadi, A., Shahabi, H., Shirzadi, A., Al-Ansari, N., Ahmad,
B. B., Chen, W., Khodadadi, M., Ahmadi, M., Khosravi, K., Jaafari, A.,
& Nguyen, H. (2020). Monitoring and assessment of water level fluctuations of the lake urmia and its environmental consequences using multitemporal Landsat 7 ETM+ images. International Journal of Environmental Research and Public Health, 17(12), 4210. https://doi.org/10.3390/ ijerph17124210
OIE. (2019a). OIE – Chapter 10.4. Infection with avian influenza viruses,
Article 10.4.1-33. Terrestrial Animal Health Code - 2019 © OIE (28/06/2019).
OIE. (2019b). Chapter 10.9. Infection with Newcastle disease virus.
Terrestrial Animal Health Code - 28/06/2019. Accessed on July
10, 2021. https://www.oie.int/fileadmin/Home/eng/Health_standards/ tahc/2018/en/chapitre_nd.pdf
OIE. (2021a). Chapter 3.3.4. Avian influenza (including infection with high pathogenicity avian influenza virus). OIE Terrestrial Manual 2021.
Accessed on July 21, 2021. https://www.oie.int/fileadmin/Home/eng/
Health_standards/tahm/3.03.04_AI.pdf
OIE. (2021b). Chapter 3.3.14. Newcastle disease (infection with Newcastle disease virus). OIE Terrestrial Manual 2021. Accessed on Nov
21, 2021. https://www.oie.int/fileadmin/Home/eng/Health_standards/ tahm/3.03.14_NEWCASTLE_DIS.pdf
Okpanachi, J. U., Umoh, J. U., Kia, G. S. N., & Dzikwi, A. A. (2020). Detection of
Newcastle disease virus (NDV) in laughing doves and the risk of spread to backyard poultry. Folia Veterinaria, 64(3), 1–12. https://doi.org/10.2478/ fv-2020-0021
Onmu¸s, O. (2008). Avian influenza monitoring in Turkey via national waterbirds census and application of GIS. In A. Demirciì, M. Karakuyu, M. A.
Mcadams, S. Incekara, & A. Karaburun (Eds.). In Proceedings of 5th International Conference on Geographic Information Systems (ICGIS), 5–8
July, Istanbul-Turkey (pp. 605–610). Istanbul, Turkey: Fatih University
Publications.
Pengra, B. (2012). The drying of Iran’s Lake Urmia and its environmental consequences. United Nations Environment Programme (UNEP) environment for development. https://na.unep.net/geas/ getuneppagewitharticleidscript.php?article_id=79. Accessed on July
2019
Piaggio, A. J., Shriner, S. A., VanDalen, K. K., Franklin, A. B., Anderson, T. D.,
& Kolokotronis, S. O. (2012). Molecular surveillance of low pathogenic avian influenza viruses in wild birds across the United States: Inferences from the hemagglutinin gene. PLoS ONE, 7(12), e50834. https://doi.org/
10.1371/journal.pone.0050834
Poulson, R. L., & Brown, J. D. (2020). Wild bird surveillance for avian influenza virus. In: E. Spackman (Ed.), Animal influenza virus. Methods in molecular biology (Vol. 2123, pp. 93–112). New York, NY: Humana. https://doi.org/10.1007/978-1-0716-0346-8_8
Rahman, A. U., Habib, M., & Shabbir, M. Z. (2018). Adaptation of Newcastle disease virus (NDV) in feral birds and their potential role in interspecies transmission. The Open Virology Journal, 12((Suppl 2),M3), 52–68. https:// doi.org/10.2174/1874357901812010052
Rehan,M., Aslam, A., Khan,M. R., Abid,M., Hussain, S., Amber, J., Anjum, A., &
Hussain, A. (2019). Potential economic impact of Newcastle disease virus isolated from wild birds on commercial poultry industry of Pakistan: A review.Hosts and Viruses, 6(1), 1–15. http://doi.org/10.17582/journal.hv/
2019/6.1.1.15
Rezaei-Far, A., Peighambari, S. M., Pourbakhsh, S. A., Ashtari, A., & Soltani,
M. (2017). Co-circulation of genetically distinct groups of avian paramyxovirus type 1 in pigeon Newcastle disease in Iran, Avian Pathology, 46(1),
36–43. https://doi.org/10.1080/03079457.2016.1203068
Sabouri, F., Vasfi-Marandi, M., & Bashashati, M. (2017). Characterization of a novel VIIl sub-genotype of Newcastle disease virus circulating in Iran.
Avian Pathology, 47(1), 90–99. https://doi.org/10.1080/03079457.2017.
1376735
Schmeller, D., Henle, K., Loyau, A., Besnard, A., & Henry, P. (2012).
Bird-monitoring in Europe – A first overview of practices, motivations and aims. Nature Conservation, 2, 41–57. https://doi.org/10.3897/ natureconservation.2.3644
Spackman, E., Senne, D. A., Myers, T. J., Bulaga, L. L., Garber, L. P., Perdue, M.
L., Lohman, K., Daum, L. T., & Suarez, D. L. (2002). Development of a realtime reverse transcriptase PCR assay for type A influenza virus and the avian H5 and H7 hemagglutinin subtypes. Journal of Clinical Microbiology,
40(9), 3256–3260. https://doi.org/10.1128/JCM.40.9.3256-3260.2002
Spackman, E., & Suarez, D. L. (2016). Avian influenza. In: S. M. Williams (Editor in Chief), Isolation, identification, and characterization of avian pathogens (6th edn., pp. 161–170). Athens, Georgia, USA: American
Association of Avian Pathologists, Inc. https://www.amazon.com/
Isolation-Identification-Characterization-Avian-Pathogens/dp/
0978916379
Spickler, A. R., Trampel, D. W., & Roth, J. A. (2008). The onset of virus shedding and clinical signs in chickens infected with high-pathogenicity and low-pathogenicity avian influenza viruses. Avian Pathology, 37(6),
555–577. https://doi.org/10.1080/03079450802499118
Ssematimba, A., Malladi, S., Bonney, P. J., Flores-Figueroa, C., MunozAguayo, J., Halvorson, D. A., & Cardona, P. I. (2018). Quantifying the effect of swab pool size on the detection of influenza A viruses in broiler chickens and its implications for surveillance. BMC Veterinary Research, 14,
265. https://doi.org/10.1186/s12917-018-1602-1
Swayne, D. E., Suarez, D. L., & Sims, L. D. (2020). Avian influenza. In: D.
E. Swayne (Editor in Chief), Diseases of poultry (14th edn., pp. 210–
256). John Willey & Sons, Inc. https://onlinelibrary.wiley.com/doi/book/
10.1002/9781119421481
Talazade, F., & Mayahi, M. (2013). Aquatic birds surveillance for Newcastle disease virus (NDV) in Khozestan Province. International Journal of Enteric
Pathogens, 01(01), 5–7. https://doi.org/10.17795/ijep9320
Trogu, T., Canziani, S., Salvato, S., Tolini, C., Grilli, G., Chiari, M., Farioli,
M., Alborali, L., Gaffuri, A., Sala, G., Bianchi, A., Rosignoli, C., Prati, P.,
Gradassi, M., Sozzi, E., Lelli, D., Lavazza, A., & Moreno, A. (2021). Survey on the presence of viruses of economic and zoonotic importance in Avifauna in Northern Italy. Microorganisms, 9(9), 1957. https://doi.org/10.
3390/microorganisms9091957
Turan, N., Ozsemir, C., Yilmaz, A., Cizmeciqil, U. Y., Aydin, O., Bamac, O. E.,
Gurel, A., Kutukca, A., Ozsemir, K., Tali, H. E., Tali, B. H., Yilmaz, S. G.,
Yaramanoglu, M., Tekelioqlu, B. K., Ozsoy, S., Richt, J. A., Iqbal, M., &
Yilmaz, H. (2020). Identification of Newcastle disease virus subgenotype
VII.2 in wild birds in Turkey. BMC Veterinary Research, 16, 277. https://doi. org/10.1186/s12917-020-02503-3
Verhagen, J. H., Fouchier, R. A. M., & Lewis, N. (2021). Highly pathogenic avian influenza viruses at the wild–domestic bird interface Europe:
Future directions for research and surveillance. Viruses, 13(2), 212. https://doi.org/10.3390/v13020212
Verhagen, J. H., Lexmond, P., Vuong, O., Schutten, M., Guldemeester, J.,
Osterhause, A. D. M. E., Elbers, A. I. W., Slaterus, R., Hornman, M.,
Koch, G., & Fouchir, R. A. M. (2017). Discordant detection of avian influenza virus subtypes in time and space between poultry and wild birds: Towards improvement of surveillance programs. PLoS ONE, 12(3), e0173470. https://doi.org/10.1371/journal.pone.0173470
Wang, C. H., Wang, Z., Lei, X., Wang, L., Li, C., Sun, Y., Wang, M., Tong, Q.,
Sun, H., & Pu, J. (2019). Infection of chicken H9N2 influenza viruses in different species of domestic ducks. Veterinary Microbiology, 233(8), 1–4. https://doi.org/10.1016/j.vetmic.2019.04.018
Wajid, A., Dimitrov, K. M., Wasim, M., Rehmani, S. F., Basharat, A., Bibi, T.,
Arif, S., Yagub, T., Tayyab, M., Ababneh, M., Sharma, P., Miller, P. J., &
Afonso, C. L. (2017). Repeated isolation of virulent Newcastle disease viruses in poultry and captive non-poultry avian species in Pakistan from
2011 to 2016. Preventive Veterinary Medicine, 142(1 July), 1–6. https:// doi.org/10.1016/j.prevetmed.2017.04.010
Wajid, A., Dundon, W. G., Hussain, T., & Babar, M. E. (2018). Pathotyping and genetic characterization of avian avulavirus-1 from domestic and wild waterfowl, geese and black swans in Pakistan, 2014 to 2017. Archives of Virology, 163(9), 2513–2518. https://doi.org/10.1007/s00705-018-
3902-y
Wajid, A., Mayahi, V., Yin, R., Ain, Q., Mohiuddin, A., Khalid, F., Rehim,
A., Manan, A., & Baksh, M. (2021). Genomic and biological characteristics of Avian Orthoavulavirus-1 strains isolated from multiple wild birds and backyard chickens in Pakistan. Tropical Animal Health and Production, 53(1), Article 90. https://doi.org/10.1007/s11250-020-
02497-y
Wille, M., & Holmes, E. C. (2020). Wild birds as reservoirs for diverse and abundant gamma- and deltacoronaviruses, FEMS Microbiology Reviews,
44(5), 631–664. https://doi.org/10.1093/femsre/fuaa026
Wise, M. G., Suarez, D. L., Seal, B. S., Pedersen, J. C., Senne, D. A., King, D.
J., Kapczynski, D. R., & Spackman, E. (2004). Development of a real-time reverse-transcription PCR for detection of Newcastle disease virus RNA in clinical samples. Journal of Clinical Microbiology, 42(1), 329–38. https:// doi.org/10.1128/JCM.42.1.329-338.2004
Zeynalova, S., Guliyev, F., Vatani, M., & Abbasov, B. (2015). Biosurveillance of avian influenza and Newcastle disease viruses in the Barda region of Azerbaijan using real time RT-PCR and hemagglutination inhibition. Frontier Microbiology, 6, 1128. https://doi.org/10.3389/fmicb.2015.
01128
Zarrineh, N., & Azari-Najaf-Abad, M. (2014). Integrated water resources management in Iran: Environmental, socio-economic and political review of drought in Lake Urmia. International Journal of Water Resources and Environmental Engineering, 6(1), 40–48. https://doi.org/10.5897/
IJWREE2012.0380
Zuhdi, S. (2017). A comparison of wildlife monitoring techniques in Riparian ecosystems of the Western United States. Master’s
Projects and Capstones, 547. https://repository.usfca.edu/capstone/
547









.jpg&w=3840&q=75)