Asian poultry production systems rapidly adopt and often adapt new health protection strategies but maximum biological performance, long-term sustainability and cost are only some of the aims of producers. It is these adaptations are the focus of this paper. New strategies are often added to previous strategies and very rarely replace or displace previous strategies and thus usually increase cost of production often with no demonstrable improvement in health status. Interference between strategies is rarely considered and lack of supporting research or critical evaluation for novel combinations is a common feature. Often the diagnostic tools needed for evaluation are readily not available. Often vaccine suppliers are also trying to promote other products in their range.
For example, mycoplasma control strategies have included mycoplasma freedom, antibiotics, killed vaccines and live vaccines. Usually, these strategies have been developed by scientific research as alternatives. Another problem is that the MG control strategy may be different from MS strategy which can cause problems in first world countries as the controls may interfere with each other. In Asia and the Middle East and some other places these strategies are often combined. Flocks may receive live mycoplasma vaccines at an early age, then during late rear, they may receive killed vaccines and finally in lay they may get antibiotics on a regular basis. All of these interventions are expensive and the return on investment should be a consideration in using the products especially in combinations where there is little scientific evidence or critical field experience to support such combinations.
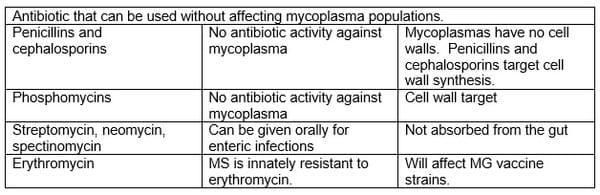
Asian poultry systems seem to be very dependent on antibiotic use. This is sometimes justified on the assumption that challenge in Asia is strong, immunosuppression is common and veterinarian supervision of antibiotic administration is different from first world production systems. It is also often justified on experience. Are there any downsides? Antibiotic resistance development can be rapid in some areas – for example, enrofloxacin has not been considered to be anti-mycoplasmal in Thailand for at least a decade (note though that the vaccine strains are still sensitive and antibiotic administration may give local field strains the upper hand). Local MG strains appeared to become resistant very rapidly. Residues of antibiotics have been an issue in Asian countries that export poultry meat but antibiotic resistance determinants as contaminants of product has yet to be an issue but is a potential future trade barrier. Audit processes currently being instigated may allow the identification of treated flocks and preclude the export of their products as being more likely to contain bacteria with transmissible resistance determinants.
This paper will consider some of the issues of “mix and match” mycoplasma control.
Live mycoplasma vaccines are inherently sensitive to antibiotics (regulators would be reluctant to register a live vaccine with acquired antibiotic resistance). Empirically ts-11 is advised to be used with no antimycoplasmal antibiotic administration for two weeks before and four weeks after vaccination and then to avoid subsequent antibiotic treatment that could affect mycoplasma infections. This latter recommendation is based on the assumption that a tracheal population only limited by the hosts immune system is needed for the maintenance of maximum mucosal immunity – that the host and the vaccine population are in balance.
Live mycoplasma vaccines are thought to work by simulating a wild-type infection. The infection of the upper respiratory tract induces mucosal immunity which then is effective at preventing further infections throughout the respiratory tract. Unfortunately, mucosal immunity has a short memory and needs constant antigen stimulation for maintenance. Fortunately, the chronic nature of mycoplasma infections is shared by live vaccines and these infect birds for life like wild strains and continue to stimulate the maintenance of mucosal immunity. Sometimes the live MG vaccine 6/85 has been criticized for failing to persist in vaccinated birds bringing its duration of immunity into doubt.
Killed vaccines produce a lot of humoral immunity which we can quantify as specific serum antibody. It is my opinion that this has very little anti-mycoplasmal effect in the trachea (or as maternal antibody in the trachea of progeny) or that killed vaccines induce very much in the way of mucosal immunity. In Asia, these vaccines are often added to live vaccine programmes, especially in non-integrated operations because the small amount of antibody that is usual from live vaccines confuses customers. The production of large uniform amounts of antibody are then argued to demonstrate that the vaccination has been done effectively (Maternal antibody may increase vertically infected embryo survival and make vertical infection of progeny flocks more efficient). If killed vaccine immunity did have an effect on mucosal populations of mycoplasma then presumably it would also affect live vaccine populations and could change the balance between host and vaccine strain and possibly make generated mucosal immunity less efficient and overall immunity less efficient than live vaccine alone.
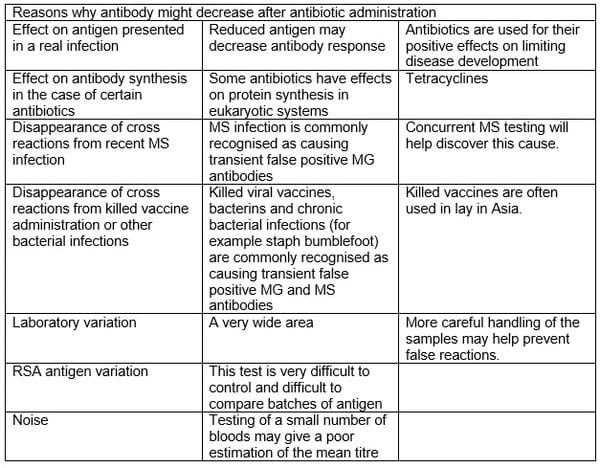
In Asia, MG antibody is often measured and summarized as percentage seropositivity or (more commonly nowadays; an ELISA mean). Some Asian vets treat flocks with antimycoplasmal antibiotics the moment they see a rise in flock antibody. The success of this intervention [assessed by the ability to prevent a further rise in mean titre] could be due to any number of reasons summarized in the table below but as most of these reasons are not real MG infections (diagnostic confirmation has been difficult to get in Asian countries) then the success of interventions in the face of challenge is probably poorly correlated.
Success breeds confidence in our actions but we need to be sure that we are not kidding ourselves.
To assess the success of a live mycoplasma vaccine one needs to know:
1) Whether the chicks being vaccinated are from MG and MS free sources.
2) The likelihood of challenge occurring before the development of immunity (three weeks after vaccination). We have strategies to overcome this problem (antibiotics in surrounding unvaccinated flocks to decrease challenge or remote rearing).
3) Whether killed vaccines were also administered.
4) Antibiotic therapy of the flock since vaccination including quinolones.
5) The clinical signs seen and which mycoplasma caused those signs.
The use of F strain in breeders is also occurs in Asia but here we have the information needed to know that it has considerable downsides. F strain’s residual pathogenicity and its regular vertical transmission into the progeny and subsequent respiratory problems that are plain mycoplasma disease. F strain would never be used in broiler or layer breeders in the USA for this reason. The pox vectored MG vaccine is also being pushed to be added to the current programmes but there are no published efficacy studies showing any benefit of this vaccine in MG control. A publication in the press by Kleven shows no benefit of this vaccine.
There is an urgent need for some controlled studies to see if these Asian practices are reducing the potential benefits of live mycoplasma vaccination. The increasing availability of live MS vaccine will make mycoplasma control easier and more efficient and producers should consider including this in their vaccination programmes. MS and MG strains can mimic each other in nearly all clinical manifestations.
It is my experience that overlaying a routine antibiotic programme on top of a live mycoplasma programme often causes problems not seen by neighboring operations successfully only using live vaccination. There is no evidence that killed vaccination is needed for biological performance reasons but sometimes commercially this is required where technical knowledge is beating against a wall of poor understanding. But even then one should consider that it may be decreasing the overall mycoplasma protection and certainly increasing production costs.
Paper presented at Poultry Health Conference, 14-15 May 2012, Bangkok Thailand.












.jpg&w=3840&q=75)