Influence of Broiler Age on the Apparent Metabolizable Energy of Cereal Grains Determined Using the Substitution Method
1. Hill, F.; Anderson, D. Comparison of metabolizable energy and productive energy determinations with growing chicks. J. Nutr. 1958, 64, 587–603. [CrossRef]
2. Mateos, G.G.; Cámara, L.; Fondevila, G.; Lázaro, R.P. Critical review of the procedures used for estimation of the energy content of diets and ingredients in poultry. J. Appl. Poult. Res. 2019, 28, 506–525. [CrossRef]
3. Wu, S.B.; Choct, M.; Pesti, G. Historical flaws in bioassays used to generate metabolizable energy values for poultry feed formulation: A critical review. Poult. Sci. 2020, 99, 385–406. [CrossRef]
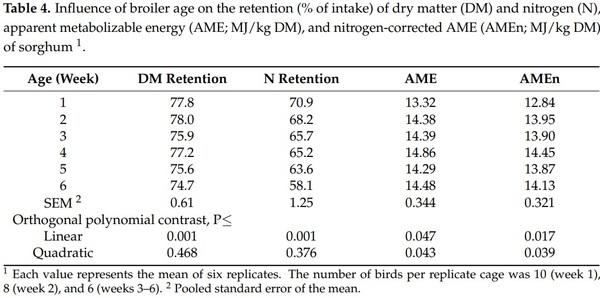
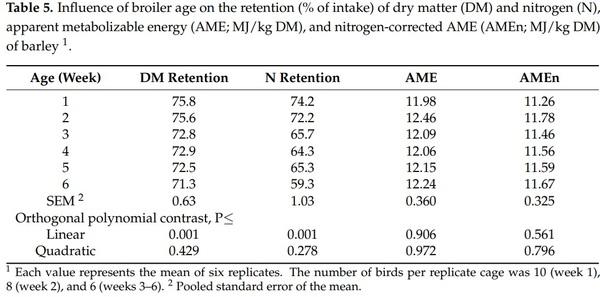
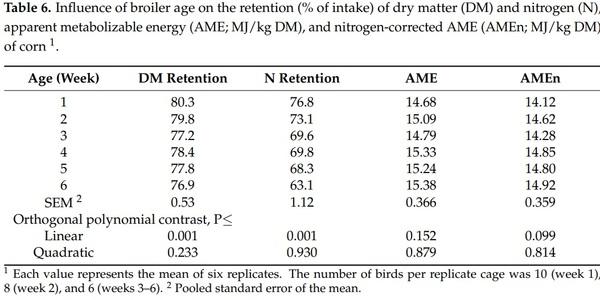
4. Khalil, M.M.; Abdollahi, M.R.; Zaefarian, F.; Chrystal, P.V.; Ravindran, V. Apparent metabolizable energy of cereal grains for broiler chickens is influenced by age. Poult. Sci. 2021, 100, 101288. [CrossRef]
5. Fraps, G.S.; Carlyle, E.C.; Fudge, J.F. Metabolizable energy of some chicken feeds. Tex. Agric. Exp. Stat. Bull. 1940, 589, 23.
6. Sibbald, I.R. A bioassay for true metabolizable energy in feedingstuffs. Poult. Sci. 1976, 55, 303–308. [CrossRef] [PubMed]
7. Sibbald, I.; Summers, J.; Slinger, S. Factors affecting the metabolizable energy content of poultry feeds. Poult. Sci. 1960, 39, 544–556. [CrossRef]
8. Farrell, D. Rapid determination of metabolisable energy of foods using cockerels. Br. Poult. Sci. 1978, 19, 303–308. [CrossRef]
9. Olukosi, O.A. Investigation of the effects of substitution levels, assay methods and length of adaptation to experimental diets on determined metabolisable energy value of maize, barley and soya bean meal. Br. Poult. Sci. 2021, 62, 278–284. [CrossRef]
10. Kong, C.; Adeola, O. Determination of ileal digestible and apparent metabolisable energy contents of expeller-extracted and solvent-extracted canola meals for broiler chickens by the regression method. SpringerPlus. 2016, 5, 1. [CrossRef] [PubMed]
11. Noblet, J.; Wu, S.B.; Choct, M. Methodologies for energy evaluation of pig and poultry feeds: A review. Anim. Nutr. 2021, 8, 185–203. [CrossRef]
12. Lockhart, W.C.; Bryant, R.L.; Bolin, D.W. A comparison of several methods in determining the metabolizable energy content of durum wheat and wheat cereal by chicks. Poult. Sci. 1967, 46, 805–810. [CrossRef]
13. Veluri, S.; Olukosi, O.A. Metabolizable energy of soybean meal and canola meal as influenced by the reference diet used and assay method. Animals. 2020, 10, 2132. [CrossRef] [PubMed]
14. AOAC International. Official Methods of Analysis, 20th ed.; Association of Analytical Chemists: Washington, DC, USA, 2016
15. McCleary, B.V.; Gibson, T.S.; Mugford, D.C. Measurement of Total Starch in Cereals Products by Amyloglucosidase vs. Amylase Method: Collaborative Study. AOAC 1997, 80, 571–579. [CrossRef]
16. AOAC International. Official Methods of Analysis, 18th ed.; Association of Analytical Chemists: Washington, DC, USA, 2005.
17. Titus, H.W.; Mehring, A.L., Jr.; Johnson, D., Jr.; Nesbitt, L.L.; Tomas, T. An evaluation of M.C.F. (Micro-Cel-Fat), a new type of fat product. Poult. Sci. 1959, 38, 1114–1119. [CrossRef]
18. SAS Institute. SAS®Qualification Tools User’s Guide; Version 9.4; SAS Institute Inc.: Cary, NC, USA, 2015
19. Lopez, G.; Leeson, S. Relevance of nitrogen correction for assessment of metabolizable energy with broilers to forty-nine days of age. Poult. Sci. 2007, 86, 1696–1704. [CrossRef] [PubMed]
20. Aderibigbe, A.; Cowieson, A.J.; Sorbara, J.O.; Adeola, O. Growth phase and dietary α-amylase supplementation effects on nutrient digestibility and feedback enzyme secretion in broiler chickens. Poult. Sci. 2020, 99, 6867–6876. [CrossRef] [PubMed]
21. Yang, Z.; Pirgozliev, V.R.; Rose, S.P.; Woods, S.; Yang, H.M.; Wang, Z.Y.; Bedford, M.R. Effect of age on the relationship between metabolizable energy and digestible energy for broiler chickens. Poult. Sci. 2020, 99, 320–330. [CrossRef]
22. Bartov, I. Differential effect of age on metabolisable energy content of high protein-low energy and low protein-high energy diets in young broiler chicks1. Br. Poult. Sci. 1995, 36, 631–643. [CrossRef]
23. Zelenka, J. Influence of the age of chicken on the metabolisable energy values of poultry diets. Br. Poult. Sci. 1968, 9, 135–142. [CrossRef]
24. Batal, A.B.; Parsons, C.M. Effect of age on nutrient digestibility in chicks fed different diets. Poult. Sci. 2002, 81, 400–407. [CrossRef]
25. Batal, A.B.; Parsons, C.M. Utilization of various carbohydrate sources as affected by age in the chick. Poult. Sci. 2004, 83, 1140–1147. [CrossRef]
26. Thomas, D.V.; Ravindran, V.; Ravindran, G. Nutrient digestibility and energy utilisation of diets based on wheat, sorghum or maize by the newly hatched broiler chick. Br. Poult. Sci. 2008, 49, 429–435. [CrossRef]
27. Tancharoenrat, P.; Ravindran, V.; Zaefarian, F.; Ravindran, G. Influence of age on the apparent metabolisable energy and total tract apparent fat digestibility of different fat sources for broiler chickens. Anim. Feed Sci. Technol. 2013, 186, 186–192. [CrossRef]
28. Lessire, M.; Leclercq, B.; Conan, L. Metabolisable energy value of fats in chicks and adult cockerels. Anim. Feed Sci. Technol. 1982, 7, 365–374. [CrossRef]
29. Scheele, C.W.; Kwakernaak, C.; van der Klis, J.D.; Bakker, G.C.M. Effects of different factors including enzymes on the nutritional value of fats for poultry. In Garnsworthy; Wiseman, J., Garnsworthy, P.C., Eds.; Recent Advances in Animal Nutrition; Nottingham University Press: Nottingham, UK, 1997; pp. 59–75.
30. Svihus, B. Limitations to wheat starch digestion in growing broiler chickens: A brief review. Anim. Prod. Sci. 2011, 51, 583–589. [CrossRef]
31. Mahagna, M.; Nir, I. Comparative development of digestive organs, intestinal dissacharidases and some blood metabolites in broilers and layer-type chicks after hatching. Br. Poult. Sci. 1996, 37, 359–371. [CrossRef]
32. Sklan, D.; Noy, Y. Hydrolysis and absorption in the small intestine of posthatch chicks. Poult. Sci. 2000, 79, 1306–1310. [CrossRef]
33. Akiba, Y.; Murakami, H. Partitioning of energy and protein during early growth of broiler chicks and contribution of vitelline residue. In Proceedings of the 10th European Symposium on Poultry Nutrition; World’s Poultry Science Association: Antalya, Turkey, 1995; pp. 44–52.
34. Noy, Y.; Sklan, D. Digestion and absorption in the young chick. Poult. Sci. 1995, 74, 366–373. [CrossRef] [PubMed]
35. Uni, Z.; Noy, Y.; Sklan, D. Posthatch changes in morphology and function of the small intestines in heavy- and light-strain chicks. Poult. Sci. 1995, 74, 1622–1629. [CrossRef]
36. Zelenka, J.; Ceresnakova, Z. Effect of age on digestibility of starch in chickens with different growth rate. Czech J. Anim. Sci. 2005, 50, 411–415. [CrossRef]
37. Lee, J.; Kong, C. Comparison of energy values estimated by direct and indirect methods for broiler chickens. Int. J. Poult. Sci. 2019, 18, 244–248. [CrossRef]
38. Zelenka, J. Effects of sex, age and food intake upon metabolisable energy values in broiler chickens. Br. Poult. Sci. 1997, 38, 281–284. [CrossRef] [PubMed]
39. Scott, T.A. Variation in feed intake of broiler chickens. Recent Adv. Anim. Nutr. Aus. 2005, 15, 237–244
40. Pettersson, D.; Aman, P. Enzyme supplementation of a poultry diet containing rye and wheat. Br. J. Nutr. 1989, 62, 139–149. [CrossRef] [PubMed]
41. Annison, G. Relationship between the levels of soluble non starch polysaccharides and the apparent metabolizable energy of wheats assayed in broiler chickens. J. Agric. Food Chem. 1991, 39, 1252–1256. [CrossRef]











.jpg&w=3840&q=75)