Feed quality and poultry gut colonisation
The Role of Feed Safety in Development of Poultry Microbiota
The first feed offered to young chicks is likely the most important meal in their life. The complex process of gut colonisation is determined with early exposure. Here we will present the role of feed quality and safety in light of the development of gut microbiota in chicks.
I. Introduction
II. Biological contaminants in feed
a) Microbial contamination of feed
b) Feed microbiota
III. Chemical contaminants in feed
c) Mycotoxins
d) Heavy metals
e) Pesticides and herbicides
f) Other feed contaminants
IV. Conclusions
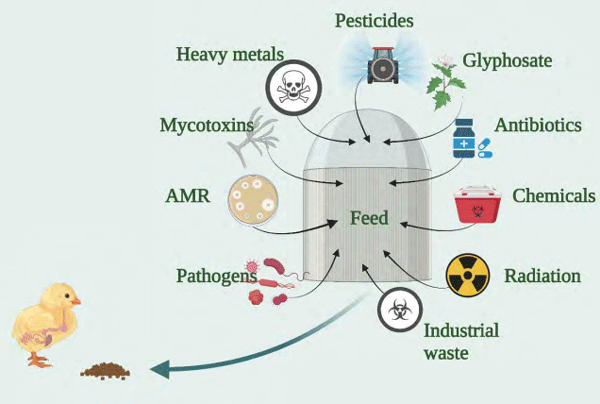
Abdallah MF De Boevre M, Landschoot S, De Saeger S, Haesaert G & Audenaert K (2018) Toxins (Basel) 10(12): 493.
Abrunhosa L, Morales H, Soares C, Calado T, Vila-Cha AS, Pereira M & Venancio A (2016) Critical Reviews in Food Science and Nutrition 56: 249-265.
Afsah-Hejri L, Jinap S, Hajeb P, Radu S & Shakibazadeh S (2013) Comprehensive Reviews in Food Science and Food Safety 12: 629-651.
Ahlawat OP, Yadav D, Kashyap PL, Khippal A & Singh G (2021) Journal of Applied Microbiology. DOI:10.1111/jam.15375
Apajalahti J, Kettunen A & Graham H (2004) World's Poultry Science Journal 60: 223-232.
Carrizo SL, Montes de Oca CE, Laino JE, Suarez NE, Vignolo G, LeBlanc JG & Rollan G (2016) Food Research International 89: 488-494.
de Boer A (2021) Current Opinion in Pulmonary Medicine 27: 263-270.
Edwards CA (2017) Annals of Nutrition and Metabolism 70: 246-250.
Eskandari MH & Pakfetrat S (2014) Food Additives & Contaminants: Part B 7: 202-207.
Gao Y, Meng L, Liu H, Wang J & Zheng N (2020) Toxins (Basel) 12(10): 619.
Gebhardt JT, Cochrane RA, Woodworth JC, Jones CK, Niederwerder MC, Muckey MB, Stark CR, Tokach MD, DeRouchey JM, Goodband RD, Bai J, Gauger PC, Chen Q, Zhang J, Main RG & Dritz SS (2018) Journal of Animal Science 96: 4149-4158.
Giller KE, Witter E & Mcgrath SP (1988) Soil Biology and Biochemistry 30: 1389-1414.
Gosling RJ, Mawhinney I, Richardson K, Wales A & Davies R (2021) Microorganisms 9.
Guerre P (2016) Toxins (Basel) 8(12): 350.
Guntinas MB, Semeraro A, Wysocka I, Cordeiro F, Quetel C, Emteborg H, Charoud-Got J & Linsinger TP (2011) Food Additives & Contaminants: Part A: Chemistry, Analysis, Control, Exposure & Risk Assessment 28: 1534-1546.
Haberecht S, Bajagai YS, Moore RJ, Van TTH & Stanley D (2020) AMB Express 10: 143.
Henderson W, Ostendorf J & Morehouse GL (1960) Avian Diseases 4: 103-109.
Holzel CS, Muller C, Harms KS, Mikolajewski S, Schafer S, Schwaiger K & Bauer J (2012) Environmental Research 113: 21-27.
Iammarino M, dell'Oro D, Bortone N & Chiaravalle AE (2015) The Italian Journal of Food Safety 4: 4531.
Islam E, Yang XE, He ZL & Mahmood Q (2007) Journal of Zheijang University Science 8: 1- 13.
Jia H, Ren H, Maita M, Satoh S, Endo H & Hayashi T (2006) Toxicology Mechanisms and Methods 16: 411-417.
Kabeer MS, Hameed I, Kashif SU, Khan M, Tahir A, Anum F, Khan S & Raza S (2021) Archives of Environmental & Occupational Health 76: 220-232.
Kaplan JL & Walker WA (2012) Current Opinion in Clinical Nutrition & Metabolic Care 15: 278-284.
Liew WP & Mohd-Redzwan S (2018) Frontiers in Cellular and Infection Microbiology 8: 60.
Makar O, Kuzniar A, Patsula O, Kavulych Y, Kozlovskyy V, Wolinska A, Skorzynska-Polit E, Vatamaniuk O, Terek O & Romanyuk N (2021) Biology (Basel) 10(5): 409.
Moebus S & Boedeker W (2021) International Journal of Environmental Research and Public Health 18(16): 8307.
Mondal NK (2020) Environmental Monitoring and Assessment 192: 590.
Muckey M, Huss AR, Yoder A & Jones C (2020) Poultry Science 99: 3841-3845.
Namulawa VT, Mutiga S, Musimbi F, Akello S, Nganga F, Kago L, Kyallo M, Harvey J & Ghimire S (2020) Toxins (Basel) 12(4): 233.
Pan D, Mionetto A, Tiscornia S & Bettucci L (2015) Mycotoxin Research 31: 137-143.
Peeters LE, Daeseleire E, Devreese M, Rasschaert G, Smet A, Dewulf J, Heyndrickx M, Imberechts H, Haesebrouck F, Butaye P & Croubels S (2016) BMC Veterinary Research 12: 209.
Pereyra CM, Cavaglieri LR, Chiacchiera SM & Dalcero AM (2011) Veterinary Research Communications 35: 367-379.
Piglowski M (2018) International Journal of Environmental Research and Public Health 15(2): 365.
Pinto B, Garritano SL, Cristofani R, Ortaggi G, Giuliano A, Amodio-Cocchieri R, Cirillo T, De Giusti M, Boccia A & Reali D (2008) Environmental Monitoring and Assessment 144: 445-453.
Pleadin J, Frece J & Markov K (2019) Advances in Food and Nutrition Research 89: 297-345.
Przenioslo-Siwczynska M, Patyra E, Grelik A, Chylek-Purchala M, Kozak B & Kwiatek K (2020) Molecules 25(9): 2162.
Richardson JB, Dancy BCR, Horton CL, Lee YS, Madejczyk MS, Xu ZZ, Ackermann G, Humphrey G, Palacios G, Knight R & Lewis JA (2018) Scientific Reports 8: 6578.
Rilstone V, Vignale L, Craddock J, Cushing A, Filion Y & Champagne P (2021) Chemosphere 282: 131048.
Robert H, Payros D, Pinton P, Theodorou V, Mercier-Bonin M & Oswald IP (2017) Journal of Toxicology and Environmental Health, Part B Crytical Reviwes 20: 249-275.
Rueda-Ruzafa L, Cruz F, Roman P & Cardona D (2019) Neurotoxicology 75: 1-8.
Ruiz Mostacero N, Castelli MV, Barolo MI, Amigot SL, Fulgueira CL & Lopez SN (2021) World Journal of Microbiology and Biotechnology 37: 14.
Sauli I, Danuser J, Geeraerd AH, Van Impe JF, Rufenacht J, Bissig-Choisat B, Wenk C & Stark KD (2005) International Journal of Food Microbiology 100: 289-310.
Schumacher LL, Cochrane RA, Huss AR, Gebhardt JT, Woodworth JC, Stark CR, Jones CK, Bai J, Main RG, Chen Q, Zhang J, Gauger PC, DeRouchey JM, Goodband RD, Tokach MD & Dritz SS (2018) Journal of Animal Science 96: 4562-4570.
Shirota K, Katoh H, Ito T & Otsuki K (2000) The Journal of Veterinary Medical Science 62: 789-791.
Sorensen MT, Poulsen HD, Katholm CL & Hojberg O (2021) Animal 15: 100026.
Stecher B & Hardt WD (2011) Current Opinion in Microbiology 14: 82-91.
Torres JP, Leite C, Krauss T & Weber R (2013) Environmental Science and Pollution Research 20: 1958-1965.
Udhayavel S, Thippichettypalayam Ramasamy G, Gowthaman V, Malmarugan S & Senthilvel K (2017) Animal Nutrition 3: 309-312.
Utembe W & Kamng'ona AW (2021) Chemosphere 271: 129817.
Utomo SW, Rahmadina F, Wispriyono B, Kusnoputranto H & Asyary A (2021) International Journal of Environmental Research and Public Health 2021: 6675374.
van der Lee MK, van der Weg G, Traag WA & Mol HG (2008) Journal of Chromatography A 1186: 325-339.
Vicini JL, Reeves WR, Swarthout JT & Karberg KA (2019) Journal of Animal Science 97: 4509-4518.
Wallace JG, Gohir W & Sloboda DM (2016) Journal of Developmental Origins of Health and Disease 7: 15-24.
Walorczyk S & Drozdzynski D (2012) Journal of Chromatography A 1251: 219-231.
Wierup M & Kristoffersen T (2014) Acta Veterinaria Scandinavica 56: 41.
Wu H, Rui X, Li W, Xiao Y, Zhou J & Dong M (2018) Food and Function 9: 2270-2281.
Wu N, Wang X, Xu X, Cai R & Xie S (2020) Ecotoxicology and Environmental Safety 192: 110323.
Xia Y, Kong Y, Seviour R, Yang HE, Forster R, Vasanthan T & McAllister T (2015) FEMS Microbiology and Ecology 91: fiv077.
Yang C, Lim W & Song G (2020) Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology 234: 108758.
Yang N, Wang H, Wang H, Wang Z, Ran J, Guo S & Peng Y (2021) Environmental Science and Pollution Research Int. DOI:10.1007/s11356-021-12958-y
Yang S, Wu Z, Lin W, Xu L, Cheng L & Zhou L (2017) Environmental Science and Pollution Research Int 24: 1372-1379.
Yasotha A, Dabade DS, Singh VP & Sivakumar T (2021) nvironmental Geochemistry Health 43: 1799-1815.
Yu Z, Gunn L, Wall P & Fanning S (2017) Food Microbiology 64: 23-32.








