INTRODUCTION
The ceca of the modern poultry bird have important implications on gut health and zootechnical performance.[1] It is estimated that cecal microbial density reaches nearly 1011–1012 cells/g.[2] The poultry cecal microflora is represented by three main phyla, the Firmicutes, Bacteroidetes, and Proteobacteria .[3]Several studies have shown theimportance of the Firmicutes in the digestion of nutrients entering the ceca.[4] It is estimated that 5%–10% of the energy from the poultry diet is extracted from cecal fermentation.[5] The undigested nutrients and non-starch polysaccharides that reach ceca are fermented by cecal microflora, resulting in the generation of volatile fatty acids (VFA’s) such as acetic acid, propionic acid, and butyric acid. These VFA’s are absorbed in the gut and contribute to energy production by way of Kreb’s cycle.[1]
Improvement in the cecal microflora to extract the undigested nutrients that reach the hind part of the intestine presents a great opportunity to improve the productivity of poultry birds. It creates an urgency to utilize the phytogenic feed additives (PFAs) which can optimize the gut health and enhance the performance of poultry birds. PFA presents an unique opportunity to intervene the dynamics of cecal microflora since several herbal extracts and essential oils have been known to modulate the gut microflora. The blend of oregano, rosemary, and fennel volatile oils in diets was reported to augment the growth and to improve the intestinal microbial balance by a reduction of coliform bacteria and an increase in Lactobacillus spp. counts of broiler chickens.[6] The researchers also observed a drastic decrease in genus Bacteroides, while certain members of order Clostridiales were increased in the cecal microbiota of chickens supplemented diets with tannins.[7] Members belonging to Clostridia have been associated with an improvement of intestinal health and feed efficiency in poultry[2]. However, most of the published studies that evaluated the influence of PFAs on gut microflora of poultry birds have utilized culture methods of identification of few selective organisms by genetic methods. These traditional methods can only reveal a part of the microflora, and there is a paucity of literature indicating the utilization of the latest tools of metagenomics such as next-generation sequencing techniques (NGS). NGS will unravel the microbiota of the gut in a comprehensive way by identifying the various communities of bacteria, some of which are not culturable in the laboratory environment.
Stodi® is a blend of several Indian medicinal plants traditionally known and being used for gut health and contains fruit rinds of Punica granatum, aerial parts of Andrographis paniculata, bark of Acacia nilotica, fruits of Terminaliabellirica, and bark of Holarrhena antidysenterica. We hypothesize the Stodi®will impact the bacterial communities in the cecum favorably for gut health to improve the performance parameters in broilers. Hence, the present study was undertaken and designed to evaluate the effect of Stodi® on cecal microflora composition in broilers using high-throughput sequencing of 16S rRNA gene amplicons. We have employed a broiler model of challenging birds with magnesium chloride hexahydrate which is known to disturb the moisture level in the cecal contents.[8]
MATERIALS AND METHODS Stodi®
Stodi® is a proprietary polyherbal formulation developed by M/s. Natural Remedies Private Limited, Bengaluru, India, containing rinds of P. granatum, aerial parts of A. paniculata, bark of A. nilotica, fruits of T. bellirica, and bark of H. antidysenterica.
Study design
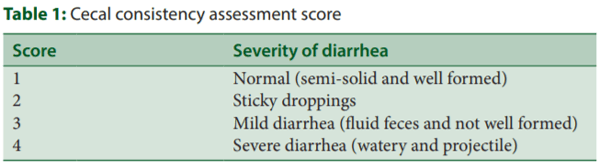
An experiment was conducted in Poultry Research Station, recognized by the Department of Scientific and Industrial Research, India; DSIR Reg No.: TU/IV-RD/2000/2016, located in Anniyalam, Tamil Nadu, India, for a period of 42 days. The chicks were housed in a semi-closed house divided into pens with floor space of 60 square feet. The approximate size of the individual pen was 6’ × 10’ × 5’ (length × width × height). Each pen was equipped with a brooder, a bell drinker, a chick feeder, and/or jumbo feeder. The size and floor space of the pen were modified according to the number of chicks housed with the help of a polyvinyl chloride sheet. The chicks were provided with poultry mash feed ad libitum[9] manufactured by Higain Feeds and Farms India Pvt. Ltd., Bengaluru (prestarter feed with 3000 kcal metabolizable energy/kg and 21% crude protein from days 1 to 10, starter feed with 3125 kcal metabolizable energy/kg and 20% crude protein from days 11 to 24 and finisher feed with 3150 kcal metabolizable energy/kg and 18.5% crude protein from days 25 to 42). A total of 960 one-day-old broiler chicks (Ross 308) purchased from Kavi Protein and Feed Pvt. Ltd., Bengaluru, were allocated equally into four groups (6 replicates/group; 40 birds/replicate) consisting of a normal diet (ND), negative control (NTC) (challenged with 1.7% magnesium chloride hexahydrate [MgCl2.6H2O]), and two treatment groups of Stodi® (S500–500 g/ton and S750–750 g/ton). NTC and treatment groups were challenged with MgCl 2.6H2O to increase the cecal moisture content as magnesium chloride is known to decrease the reabsorption of water in the cecum and subsequently to disturb the cecal microbiota. All birds appeared healthy throughout the experimental period and were individually weighed on days 1 and 42. The body weight gain and the feed conversion ratio (FCR: ratio of feed intake/body weight) were calculated on day 42. Ten chickens per group were randomly selected on day 43 and sacrificed by jugular vein exsanguination. The cecal samples were collected in Petri plates and the cecal consistency was assessed and scored as per Table 1.
Sample collection and DNA extraction
Ten chickens per group were randomly selected on day 43 and sacrificed by jugular vein exsanguination. The cecal contents were aseptically collected in the DNA/RNA Shield™ Fecal Storage Tube (Zymo Research, CA, USA) and stored at room temperature until DNA extraction. Total DNA was isolated from cecal contents using DNeasy® Blood and Tissue kit (Qiagen, Valencia, CA, USA) as per the manufacturer instructions. DNA concentration and quality were assessed in NanoDrop ND-2000 spectrophotometer (NanoDrop Technologies, DE, USA). DNA was stored at −80°C until further analysis.
16S rRNA gene library preparation and high-throughput sequencing
The 16S rRNA gene V3-V4 regions were amplified using Illumina primers (forward 5’-AATGATACGGCGACCACCGAGATCTACA C[i5]TCGTCGGCAGCGTC-3’, reverse 5’-CAAGCAGAAGACGGCAT ACGAGAT[i7]GTCTCGTGGGCTCGG-3’) with standard adapter sequences attached for barcoding and multiplexing. Nextera DNA libraries were made for each of the five pooled samples per group (two samples per pool in equimolar concentrations) separately using Nextera™ DNA Library Preparation Kit and Nextera™ Index Kit (Illumina, San Diego, CA, USA) as per the manufacturer instructions. Paired-end sequencing (2 bp × 300 bp) was conducted on an Illumina MiSeq 500 sequencing platform.
Microbial community analysis
The Illumina paired-end V3-V4 reads (2 bp × 300 bp) were demultiplexed using bcl2fastq1 tool and quality checked using FastQC.[10] The raw reads having primer sequence and high-quality bases were selected and further stitched using Fastq-join3. Microbial composition and diversity were analyzed in the stitched reads using Quantitative Insights into Microbial Ecology (QIIME)[11] pipeline. Open-reference Operational Taxonomic Units (OTUs) picking was performed using UCLUST[12] method against a curated chimera free 16S rRNA database (Greengenes[13] v 13.8). The taxonomies were assigned using RDP[14] classifier to these clusters at ≥ 97% sequence similarity against the reference database which resulted in the generation of a biom file. The biom was taken ahead for further advance analysis and visualization. To standardize sequence counts across samples, 130,000 sequences were selected per sample (rarefaction) and used as a basis to compare abundances of OTUs across samples. Alpha diversity was calculated by observed OTUs, Shannon Index, Simpson Index, and Chao Index. Principal coordinates analysis (PCoA) plot were computed based on weighted and unweighted UniFrac distances. This method is a beta diversity measure, termed as pair-wise analysis, that considers the phylogenetic divergence among OTUs to identify differences in the overall microbial community structure between the samples.
Statistical analysis
The relative abundances of bacterial populations were analyzed using GraphPad Prism Software, CA, USA.[15] Relative abundances at each level of classification (phylum, class, order, family, and genus) were compared by one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test and growth performance parameters was compared by one-way ANOVA followed by Least Significant Difference test, which were considered statistically significant when P < 0.05. Krona charts were generated using Krona tools[16] which gives the quantitative phylogenetic information for all the samples. PCoA is constructed using QIIME[11] pipeline.
RESULTS
Alpha diversity
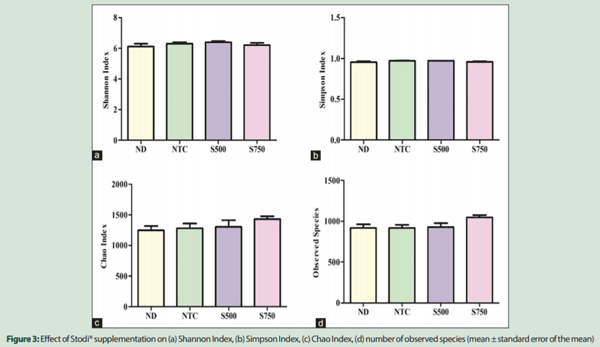
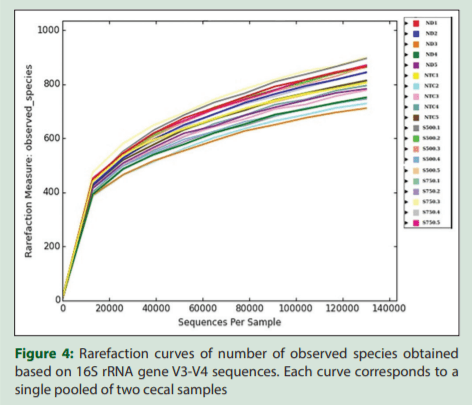
Internal sample alpha diversity was estimated through the number of observed species, Simpson, Chao, and Shannon indices (diversity). There was no statistical difference between the groups with respect to the number of observed species, estimated richness (Chao), or diversity (Simpson and Shannon indices) [Figure 3]. Rarefaction curves of observed species reached a plateau in all samples, indicating that the read depth was sufficient to cover the microbial diversity in poultry cecal samples [Figure 4].
Performance traits
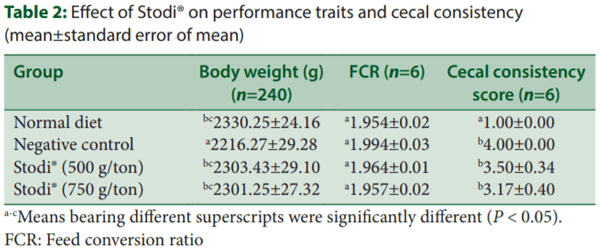
All chicks were healthy before initiation of the experiment. The addition of magnesium chloride caused a significant reduction in body weight (P < 0.05) and worsened the FCR in NTC group on day 42. However, dietary addition of Stodi® (500 and 750 g/ton) improved the body weight and FCR on day 42 when compared to NTC group. The cecal consistency score was significantly increased in NTC as compared to ND, whereas S500 and S750 decreased the cecal consistency score as compared to NTC [Table 2].
Sequencing data
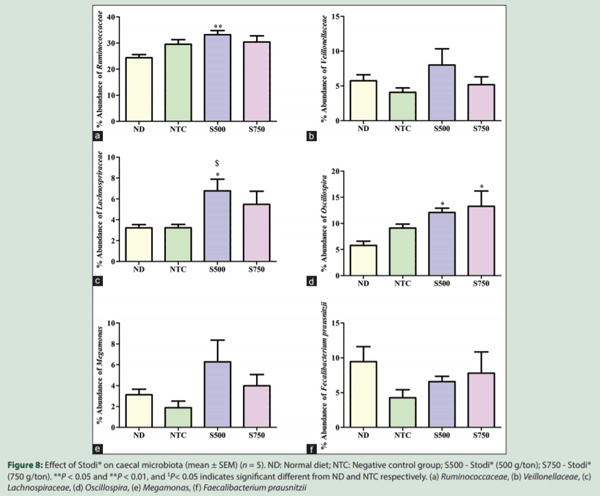
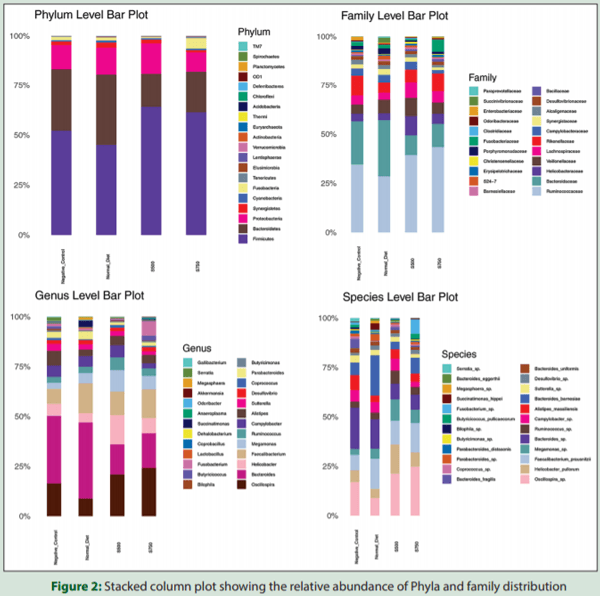
A total of 8,690,663 paired-end reads were obtained from 20 pooled cecal samples. After quality filtering and removal of chimeric reads, the average clean tags obtained for the ND, NTC, S500, and S750 were 228606, 235133, 188960 and 297959 respectively. The average number of reads per cecal sample was 425836. An average of 952 OTUs with abundance >0.005% were obtained from all samples. At the phylum level, Firmicutes, Bacteroidetes, and Proteobacteria accounted for more than 85% of cecal microbiota, in which Firmicutes (40%– 65%) and Bacteroidetes (20%–35%) were the predominant microbes followed by Proteobacteria (9%–14%) in all the sequences. Firmicutes alone was represented by eight genera, but only Oscillospira, Faecalibacterium, and two unclassified genera each represented morethan 5% of all the sequences of this phylum. Other genera included Megamonas, Ruminococcus and two unclassified genera representedby more than 1% of the total bacterial sequences. Bacteroides was the most predominant genus, accounting for 50%–80% of the sequences in the phylum Bacteroidetes. Other relatively predominant genera in this phylum included Parabacteroides and Alistipes. Campylobacter,Helicobacter, Sutterella and Desulfovibrio were the most predominantgenera in the phylum Proteobacteria. Furthermore, Krona chart is an interactive plot used to explore the relative abundances and confidences within the complex hierarchies of metagenomes classifications. In the present study, Stodi® treatment produces an impact on percentage of Firmicutes and Bacteroidetes ratio which was presented visually in one representative Krona chart [Figure 1]. In addition, stacked column plots were generated for the top 26 unique classified organisms identified at phylum, family, genus, and species taxonomic level using relative abundance values for all the samples [Figure 2].
Alpha diversity
Internal sample alpha diversity was estimated through the number of observed species, Simpson, Chao, and Shannon indices (diversity). There was no statistical difference between the groups with respect to the number of observed species, estimated richness (Chao), or diversity (Simpson and Shannon indices) [Figure 3]. Rarefaction curves of observed species reached a plateau in all samples, indicating that the read depth was sufficient to cover the microbial diversity in poultry cecal samples [Figure 4].
Beta diversity
PCoA based on weighted and unweighted UniFrac distances was performed to determine the formation of clusters according to the treatments [Figures 5 and 6]. Although sample overlapping occurred between the four sample groups, clustering tendencies were observed, suggesting that the samples of each treatment inclined to form separate series, indicating the S500 and S750 differentially modulate the cecal microbiota in comparison with NTC and ND groups.
Effects of Stodi® on composition of cecal microbiota
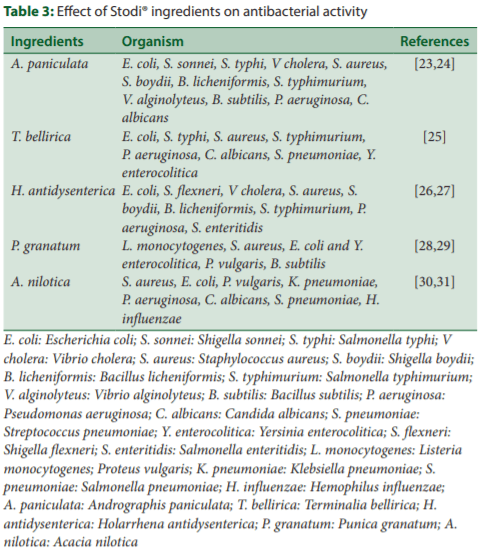
In S500 and S750 groups, the Firmicutes in the cecum was more abundant (P < 0.05), while the Bacteroidetes was less abundant (P < 0.05) as compared to ND group. In addition, Bacteroidetes in the cecum was less abundant (P < 0.05) in S500 group as compared to NTC group. The ratio of the two-predominant phyla, Firmicutes and Bacteroidetes (FBR) did not differ significantly between the groups (P > 0.05). However, FBR was numerically improved in both S500 (P < 0.05 vs. ND only) and S750 group as compared to ND and NTC groups [Figure 7].
At the class level, Clostridia (P < 0.05 vs. ND only) and Bacilli were relatively more abundant in S500 and S750 as compared to ND and NTC groups, whereas Erysipelotrichi was relatively more abundant in S500 and S750 as compared to ND group [Figure 7].
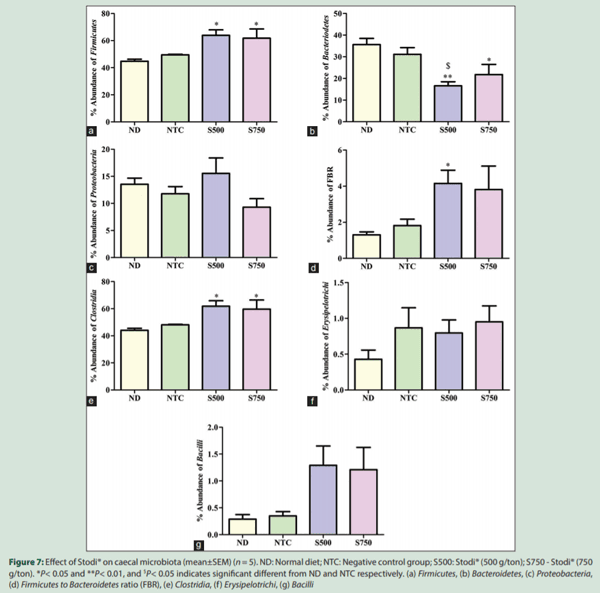
At the family level, the abundance of Ruminococcaceae was increased in S500 (P < 0.05) and S750 as compared to ND group, whereas the abundance of Lachnospiraceae was increased in S500 (P < 0.05) and S750 as compared to ND and NTC groups. Similarly, the abundance of Veillonellaceae was greater in S500 and S750 than NTC group [Figure 8].
At the genus level, S500 and S750 showed a higher abundance of Oscillospira as compared to ND (P < 0.05) and NTC groups. Similarly,the abundance of Megamonas was numerically improved in S500 and S750 groups as compared to ND and NTC groups. At the species level, Faecalibacterium prausnitzii was relatively more abundant in S500 andS750 as compared to NTC group [Figure 8].
DISCUSSION
There is a constant pressure to upgrade the productivity of poultry birds to compensate for the increasing feed costs and bird management practices. The gastrointestinal tract of broiler chickens occupies a pivotal role in utilizing the nutrients effectively for growth and contributing to the productivity of the poultry. Specifically, the cecal microbiota of modern poultry birds plays a critical role by harvesting the energy from the diet. The traditional culture-based techniques enable the recovery of microbial isolates for characterization under both anaerobic and aerobic conditions. However, <60% of the bacterial population of the poultry intestine are estimated to be cultivable under standard laboratory conditions.[2] Hence, the complexity of the gut microflora has been fairly understood by utilizing the latest techniques of molecular biology, namely, NGS techniques. In the present study, the NGS revealed a wide variety of microorganisms in the cecum of the Ross 308 broiler chickens and the impact of a polyherbal formulation, Stodi® on cecal microbiome was evaluated.
The results of the present study revealed three important phyla in the ceca, namely, Firmicutes, Bacteroidetes, and Proteobacteria in the broiler birds and were in accordance with the previous studies.[4,17] Moreover, the FBR was elevated upon supplementation with Stodi® at both doses but significantly at S500 (500 g/ton of feed) and is positively correlating with improved body weight and FCR of broiler birds. A wide variety of growth promoters increase the FBR, for example, antibiotic growth promoters,[3] plant extracts,[18] and prebiotics.[19] The effect of polyphenolic compounds from blueberry and blackberry have been shown to improve the performance and FBR in the ceca of broilers.[18] The Stodi® contains phytoconstituents such as polyphenols (gallic acid, catechins, punicalagins from P. granatum, T. bellirica, A. nilotica, and H. antidysenterica) and diterpenoid lactone (andrographolide from A. paniculata). The observed improvement in FBR and performance couldbe attributed to the polyphenolic constituents of Stodi®.
The predominant phylum increased upon Stodi® supplementation was Firmicutes. These organisms play a vital role in fermentation of dietarysubstrates, transporting many substances across cellular membranes. It has been suggested that Firmicutes dense ceca increases the capacity of the host to extract energy from the diet, with carbohydrate utilization being the important key element.[4] The cecal Firmicutes are known to be specifically involved in the production of butyrate as well.[20] The Firmicutes are predominantly represented by three classes, namely, Clostridia, Erysipelotrichi, and Bacilli. The most abundant class that wasrepresented by all treatments were Clostridia which was significantly increased by both doses of Stodi®. The genus Clostridium is reputed in the poultry industry, mainly because of a harmful species, Clostridiumperfringens. It is important to note that Clostridium is just one ofthe 123 genera belonging to the order Clostridiales under the family Clostridia. Furthermore, the species C. perfringens is only remotelyrelated to the 129 species within the genus Clostridium. Most of the Clostridia are very beneficial to gut health and are associated witheconomic impact.[2]
The family Ruminococcaceae under the order Clostridiales was significantly increased by Stodi® at 500 g/ton of feed. This family is involved in fostering the performance of the broiler birds mainly by the way of production of short-chain fatty acids (SCFA’s) and degradation of plant materials.[21] Similarly, the family Lachnospiraceae was increased by Stodi® at a dose of 500 g/ton. The members of Lachnospiraceae are correlated with increased energy production by releasing SCFA’s and improving gut health.[21]
Stodi® had an interesting impact on the genus Oscillospira. Both doses of Stodi®, dose dependently increased the abundance of this genus in a statistically significant manner. Oscillospira, a genus under the family Ruminococcaceae, is an important organism in the ceca for energymetabolism and is involved in the fermentation of various mono- and oligo-saccharides, which results in the generation of SCFA’s that contributes to the energy production[22] and promotion of gut health as well. Stodi® did not produce significant impact on Proteobacteria although there was nonsignificant reduction in abundance of this phylum at the dose of 750 g/ton of feed. There was also a reduction in family Enterobacteriaceae upon Stodi® supplementation at both doses, although this was not statistically significant.
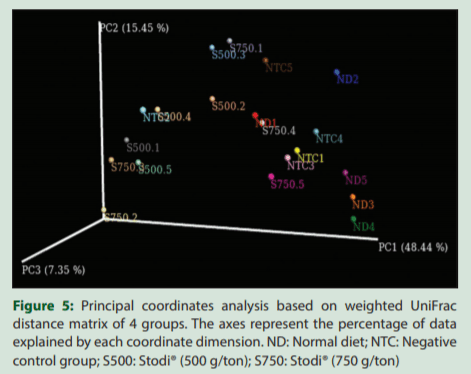
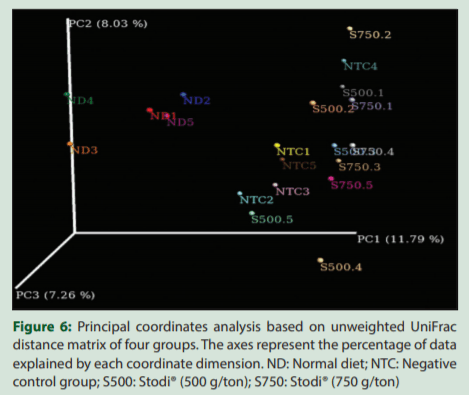
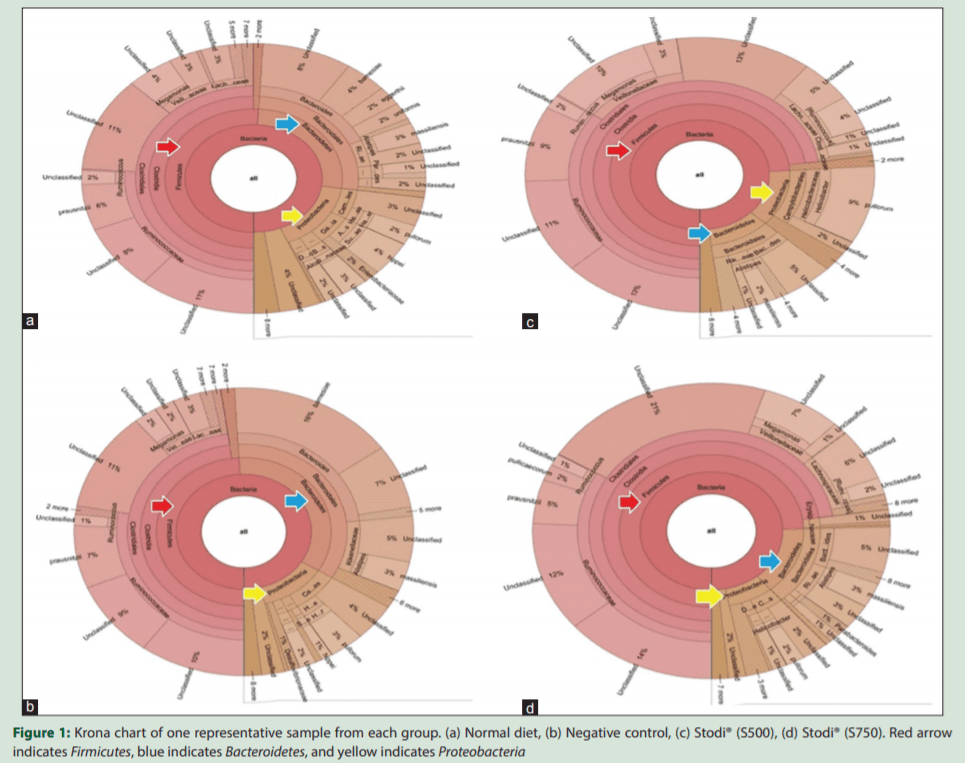
The beta-diversity analysis revealed that the Stodi® treatment produced a distinct microbiota shift in comparison with ND and NTC. However, the beta-diversity between the treatments of two doses of Stodi® was small, indicating that both doses produce a shift in the microflora in analogous manner. In general, the digestion in the fore and midgut influences the microflora of the hindgut.[2] Furthermore, the type and quantity of nutrients reaching the hindgut and ceca would have been influenced by Stodi® supplementation, as the different ingredients of Stodi® have been reported to have antibacterial effects [Table 3]. Andrographolide, being one of the active constituents of Stodi®, has been reported to improve the activity of brush border enzymes such as maltase and sucrose.[32] These effects would have impacted the metagenomic profile of ceca of Stodi®-treated groups.
The findings of the present study also indicate, for the first time, the PFA Stodi® produces a shift in the cecal microbiota favorable for better gut health and improves the FBR and subsequently the body weight and FCR in broiler chickens. The improvement in the Firmicutes abundance caused by Stodi® could have resulted in better energy extraction from the utilization of carbohydrates and butyrate production. The improvement in Ruminococcaceae and Lachnospiraceae by Stodi® could have resulted in better fermentation of various mono- and oligo-saccharides, resulting in the generation of SCFA’s and contributing to the energy production and promotion of gut health and better performance traits in broiler chickens.
CONCLUSION
The supplementation of Stodi® in the diet of broilers produced a shift in the cecal microbiota conducive for the gut health and better performance characteristics. Stodi® caused a favorable improvement in Firmicutes, specifically Clostridiales and more specifically Ruminococcaceae and Lachnospiraceae, and subsequent better utilization of carbohydratesand production of SCFA’s contributing to energy production and gut health.
Acknowledgements
The authors are thankful to the Managing Director, M/s. Natural Remedies Private Limited, Bengaluru, India, for providing facilities and infrastructure to carry out the study. The authors gratefully thank all of the staff at the Genotypic Technology Pvt. Ltd., for metagenomic analysis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.