Manipulating the immune system of layers and breeders: novel applications of mannan oligosaccharides
Two immune systems exist at least operationally in avians and mammals. The innate system provides early protection and is said to be nonspecific. Acquired immunity has specificity and is thought to act later during the course of infection. Some of the differences between susceptibility and resistance depend on genetics. As a result, success will depend on identifying the functions of as many of the genes as possible and the ways through which they carry out their responsibilities. Better integration of the two systems is the overall objective. Pitting one against the other would be counterproductive.
Currently vaccines are used widely in poultry while antibiotics are becoming restricted. Improvement of the former or developing alternatives for the latter seems to be the logical course. Finding growth enhancers also having a positive effect on immunity would be advantageous.
Savage et al. (1996) and Savage and Zakrzewska (1996) showed that phosphorylated mannan oligosaccharide (Bio-Mos®) elevated plasma IgG and IgA in turkeys. Pullets fed Bio-Mos® had a milder cutaneous response to the toxic lectin phytohemagglutinin (PHA) (Cotter, 1997). Since this is a form of hypersensitivity, the combined evidence shows that Bio-Mos® may affect humoral and cellmediated immunity. It is likely that Bio-Mos® or related substances have the potential to alter immune parameters in ways that are as yet undefined. Uncovering these properties seems warranted, especially in commercially important stock reared under near field conditions.
Experimental strategies
Chickens immunized with heterologous erythrocytes produce the corresponding agglutinating antibody (Delhanty and Solomon, 1966; Seto and Henderson, 1968). Immunizations with soluble proteins produce precipitating antibody (Wolf, 1942). These antigens model how chickens respond to infectious microbes. Additionally their use circumvents some of the difficulties associated with pathogenic challenge protocols.
Chickens produce high titer (natural) antibody to various environmental antigens. Those expressing galactose (‘α-gal epitope’, Galili et al., 1984) induce the production of antibody that cross-reacts with rabbit erythrocytes (Bailey, 1923; Cotter, 1998). This natural antibody is easily measured because it agglutinates rabbit red blood cells. Combining this strategy with growth or production experiments affords the opportunity to obtain information on both innate and acquired immunity. Serum samples collected during the course of such experiments will contain the two types of antibody. Measuring changes in their levels could show how production and immunity are coupled.
This report will focus on how dietary phosphorylated mannan oligosaccharide (Bio-Mos®) affected both natural (anti-gal) and acquired antibody (SRBC, BSA) titers. Both commercial laying hens and broiler breeders were studied. No attention will be given here as to how Bio-Mos® affects production; for the latter the reader is directed to other reports presented in this or earlier volumes.
CHICKENS AND IMMUNIZATION
Experiment 1: commercial layers. A total of about 100 commercial laying hens (DeKalb W-36) housed in individual cages were placed on four (corn/soy) diets containing 0, 0.05, 0.1, or 0.2 % Bio-Mos®. After four weeks on the test diets, each hen was sensitized with an intraperitoneal injection of sheep red blood cells (SRBC) and 10% bovine serum albumin (BSA) in PBS. Serum samples were obtained from wing vein blood drawn on weeks 0 (pre-immune), 1, 2 and 4 post-sensitization. Obtaining multiple samples allowed the time course (kinetics) of the responses to be followed.
Experiment 2: broiler breeders. About 100 broiler breeder hens (Hubbard) were fed the same diets as described for Experiment 1. There were two pens per dietary treatment and 12-13 hens per pen. All were fed at 0.35 lbs/hen/day. Hens were immunized with SRBC without BSA and bled using the same schedule as for the layers. All sera were stored frozen and later tested for the corresponding antibody.
ANTIBODY TESTING
Standard microtiter hemagglutination procedures were used to determine SRBC and RRBC (antigal) titers (Wegmann and Smithies, 1966). BSA titers were determined using passive hemagglutination (PA) by microtiter. The antigen (BSA) was conjugated to chicken cells (the carrier) with chromic chloride (Bigazzi et al. 1986). This step was used to assure specificity, as any crossreaction to mammalian erythrocytes (due to natural antibody) was avoided. BSA is a soluble protein, so precipitating antibody is the normal result (Wolfe, 1942). Thus the PA technique used here allowed BSA antibody to be detected by the more sensitive agglutination method.
Results
The results of the experiments will be presented according to antibody. In Experiment 1 SRBC and BSA antibodies measure acquired immunity, while the anti-gal response (measured using rabbit erythrocytes) represents innate immunity. Since the broiler breeders (Experiment 2) were not deliberately immunized with BSA, the development of these antibodies technically cannot represent acquired immunity. However, since their levels increased and decreased with the SRBC response, they followed kinetics typical of an acquired response. Their assignment to one or the other category is thus problematic and reflects the sometimes arbitrary distinction between the two immune systems.
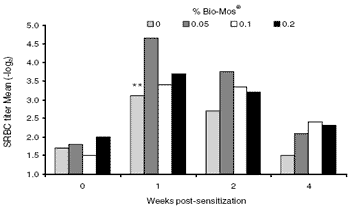
Figure 1. The SRBC response kinetics in commercial layers. Titers were obtained by diluting sera from about 25 hens per treatment with PBS. There was a statistically significant difference (**) between dietary treatments for week 1.
The kinetics (time course) of the SRBC response are shown by Figure 1. The highest titers occurred one week post–immunization with declining levels thereafter. These results are typical of an acquired immune response. The enhancing effect of Bio- Mos® on these antibodies is also shown in the same figure. It is clear that Bio-Mos®-fed hens produced higher levels of SRBC agglutinins over the course of the trial. The difference was only statistically significant (P< 0.01) at week 1, while numerically higher levels were seen at weeks 2 and 4. It was interesting that those hens fed the lowest level of Bio-Mos® (0.05%) had higher titers than hens in the other two Bio-Mos® treatments.
The kinetics of the SRBC response of broiler breeders paralleled those of the layers (Figure 2).
All treatment groups had their maximum response at week 1, followed by declining titer levels at two and four weeks post-sensitization. The effect of Bio- Mos® in broiler breeders contrasted somewhat with that seen in the layers. A higher level of SRBC antibody was found in breeders fed the Bio-Mos® diets, but it was not significantly different from the controls. During weeks 1 and 2 post-immunization, however, there was a tendency for higher titers to be found at the higher levels of Bio-Mos® inclusion. SRBC antibody appeared slightly higher in broiler breeders compared with layers at all measurement times including the ‘pre-immune’ levels. The difference may be related to the strains used in each experiment or to housing (floor versus cage).
It is important to note that the chickens used in both experiments responded to SRBC immunization even though pre-immune antibody was present. Thus, the presence of pre-sensitization antibody (presumably natural antibody) did not interfere with the ability to produce the acquired antibody. Additionally the response levels of the two experiments were similar in being about three logs above the pre-immune level.
Taken together, the results of the two experiments demonstrated that including Bio-Mos® in the diets resulted in increased levels of SRBC agglutinins. From a practical viewpoint this would translate into the likelihood that immunity to pathogens might also be enhanced. Phagocytic responses that depend on opsonizing antibody would be good candidates for further investigation.
The effects of Bio-Mos® on BSA and anti-gal antibody levels will be considered below as part of the discussion.
Discussion
The capacity of Bio-Mos® to affect humoral immune parameters was reported by Savage and Zakrzewska (1996), who found higher globulin levels in turkeys fed diets that included Bio-Mos®. Cotter (1997) showed that Bio-Mos®-fed hens had less wattle swelling hypersensitivity than controls. Therefore, their immunologic injury (hypersensitivity) was reduced. Experiments showing improved Newcastle and bronchitis antibody titers in chickens exposed to mycotoxins were reviewed by Devegowda et al. (2000). Clearly, the accumulated evidence demonstrates that Bio-Mos® affects both arms of the immune response.
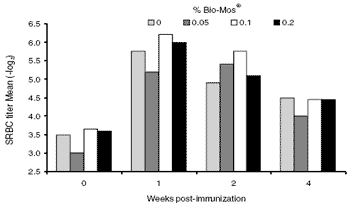
Figure 2. The SRBC response kinetics of broiler breeders. Titers were obtained by diluting sera from about 25 hens per treatment with PBS.
In the studies reported herein, both layers and broiler breeders had low levels of preexisting (natural) SRBC antibody. Immunization increased SRBC titer with peak responses occurring at one week followed by steady declines thereafter. The abrupt rise and subsequent decline suggests that this is due to production of immune antibody superimposed on preexisting natural antibody.
The titer of SRBC antibody was elevated over the controls in the layer hens fed the lowest level of Bio-Mos® (0.05 %), especially at week 1. A similar trend was observed in the broiler breeders, but the response was more dependent on the concentration of Bio-Mos® included in the diet. These differences may arise from a myriad of causes, but they highlight the need for tailoring the amounts of Bio-Mos® to specific situations.
Low levels of BSA antibody existed in the layers prior to immunization. These rose after immunization and were slightly higher in Bio-Mos®-fed hens, but the difference was not significant. Again, the rise and fall in titer suggests these were immune antibodies. Perhaps because BSA is so powerful an immunogen in chickens (Wolfe, 1942) the difference due to the presence of Bio-Mos® was slight (Figure 3).
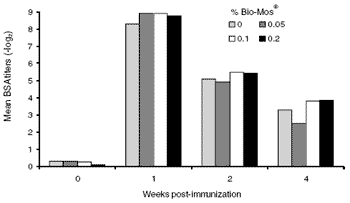
Figure 3. BSA response kinetics in commercial layers measured by the passive hemagglutination (PA) technique. Low levels of pre-immune BSA antibody were found at week 1.
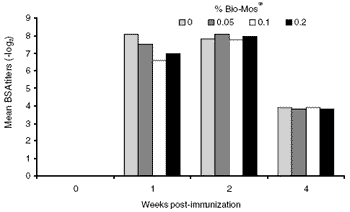
Figure 4. BSA response kinetics in broiler breeders measured by the passive hemagglutination technique. No BSA antibodies were detected prior to SRBC immunization.
Interestingly, anti-BSA titers appeared in the broiler breeders subsequent to SRBC injection. Although none were detected in pre-immune sera, they rose and declined in parallel with the changes in the SRBC response (Figure 4). They cannot be a response to dietary beef by-products or a result of BSA exposure during vaccination, as neither was used. The diets did contain 1.3% pork bone meal, however, so these may be cross-reactive with pork specificities, but their absence prior to SRBC immunization makes this possibility doubtful. They may arise as part of the general inflammatory response associated with SRBC injection. Identifying the role such antibody plays in immunity should be a focus of future experiments.
Rabbit antibody (anti-gal) is naturally-occurring in chickens (Bailey, 1923; Cotter, 1998). Its presence is believed to result from environmental stimuli. Galactose residues on microbial surface structures are thought to be an important stimulus (Galili, 1994).
Kinetic differences in anti-gal titers were evident between the two experiments. They were higher in the layers than in broiler breeders to begin with (Figures 5 and 6, left). They rose on week 1 in both groups but followed different courses in the two experiments. They declined in layers after week one but they continued to rise through week four in broiler breeders. Since the source of this antibody is microbial or viral in origin, some of the difference might arise as a result of temporary changes in gut flora coincidental to antibody production or to other physiologic events that were peculiar to each experiment. No statistically significant effects of diet treatments on the anti-gal antibody were evident in broilers.
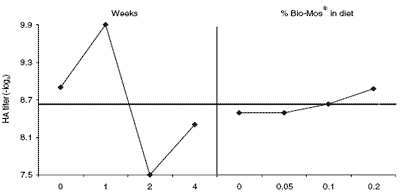
Figure 5. Main effects plots (least squares means) of the natural anti-gal antibody in commercial layers. The response kinetics, ignoring the diet treatments, is on the left. The right shows diets but ignores weeks. The broken line represents the mean for all.
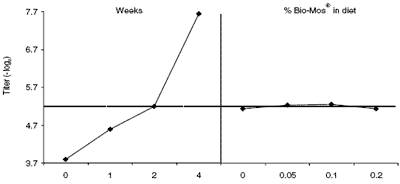
Figure 6. Main effects plots (least squares means) of the natural anti-gal antibody in broiler breeder hens. The response kinetics, ignoring the diet treatments, is on the left. The right shows diets but ignores weeks. The broken line represents the mean for all measurements.
The mechanisms responsible for all of the observed effects of Bio-Mos® on immunity are unknown. It has binding capacity for certain enteric pathogens (Spring et al., 2000) and it adsorbs potentially immunosuppressive mycotoxins (Stanley et al., 1993). Either of these properties may account for part of the current observations. However, the present results indicate that there are additional immunomodualtory properties possessed by Bio- Mos®.
Mannose-binding lectin (MBL), an opsonin that aids in phagocytosis, is a minor acute phase protein in chickens (Nielsen et al., 1999). It participates in the innate immune response to microorganisms. Perhaps the surface mannose present on Bio-Mos® stimulates MBL production. If so, this may have aided the processing of SRBC for presentation to T-cells, a necessary prerequisite for the production of acquired antibody.
In the layers there may have been a temporary redirection of antibody synthesis away from antigal toward SRBC. This might represent a form of ‘immunologic homeostasis’ (Cotter, 2001) in which the innate system provides reserves that bridge with acquired immunity. This seemed not to be the case in broiler breeders, as the anti-gal titers continued to rise. In any event, it is now quite clear that Bio- Mos® assists immunity by several mechanisms beyond pathogen binding and mycotoxin adsorption. These properties seem to mimic some of the benefits earlier observed for antibiotics without having their downside.
References
Authors: P.F. COTTER1, A.E. SEFTON2 and M.S. LILBURN3Bailey, C.E. 1923. A study of the normal and immune hemagglutinins of the domestic fowl with respect to their origin, specificity and identity. J. Hygiene 3:370-393.
Bigazzi, P.E., C.L. Burek and N.R. Rose. 1986. In: Manual of Clinical Immunology, 3rd ed. (N.R. Rose, H. Friedman and J.L. Fahey, eds.) American Society for Microbiology, Washington D.C. pp. 762-770.
Cotter, P.F. 1997. Modulation of the immune response: current perceptions and future prospects with an example from poultry. In: Biotechnology in the Feed Industry, Proceedings of the 13th Annual Symposium, (T.P. Lyons and K.A. Jacques, eds). Nottingham University Press, Nottingham, U.K. Pp 195-203.
Cotter, P.F. 1998. Naturally occurring rabbit erythrocyte agglutinins in fowl sera. Poultry Sci. 77(Suppl. 1) 100.
Cotter, P.F. and S. Halidi. 2001. Wattle swelling and antibody titers in BSA hypersensitive and naive hens. Poultry Sci. 80(Suppl. 1) 129.
Delhanty, J.J. and J.B. Solomon. 1966. The nature of antibodies to goat erythrocytes in the developing chicken. Immunology 11:103-113.
Devegowda, G., M.V.L.N. Raju, R. Afzali and H.V.L.N. Swamy. 2000. Mycotoxin picture worldwide: novel solutions for their counteraction.
In: Biotechnology in the Feed Industry, Proceedings of the 14th Annual Symposium, (T.P. Lyons and K.A. Jacques, eds). Nottingham University Press, Nottingham, U.K. pp 241-255.
Galili, U.E., A. Rachmilewitz, A. Peleg and I. Flechner. 1984. A unique natural human IgG antibody with α-galactosyl activity. J. Exp. Med. 160:1519-1531.
Nielsen, O.L., J.C. Jensenius, P.H. Jorgensen and S.B. Laursen. 1999. Serum levels of chicken mannan-binding lectin (MBL) during virus infections; indication that MBL is an acute phase reactant. Vet. Immunol. Immunopathol. 70:309- 316.
Savage T.F., P.F. Cotter and E.I. Zakrzewska. 1996. The effect of feeding mannanoligosaccharide on immunoglobulins, plasma IgG and bile IgA, of Wrolstad MW turkeys. Poult. Sci. 75(Suppl. 1):143.
Savage T.F. and E.I. Zakrzewska. 1996. The performance of male turkeys fed a starter diet containing a mannanoligosaccharide (Bio-Mos®) from day old to eight weeks of age. In: Biotechnology in the Feed Industry, Proceedings of the 12th Annual Symposium, (T.P. Lyons and K.A. Jacques, eds). Nottingham University Press, Nottingham, U.K. pp 47-54.
Seto, F. and W.G. Henderson. 1968. Natural and immune hemagglutinin forming capacity of immature chickens. J. Exp. Zool.169:501-512.
Spring P., C. Wenk, K.A. Dawson and K.E. Newman. 2000. The effects of dietary mannanoligosaccharides on cecal parameters and the concentration of enteric bacteria in the ceca of salmonella-challenged broiler chicks. Poult. Sci. 79:205-211.
Stanley, V.G., R. Ojo, S. Woldesenbet and D.H. Hutchinson. 1993. The use of Saccharomyces cerevisiae to suppress the effects of aflatoxicosis in broiler chicks. Poult. Sci. 72:1867-1872.
Wegmann, T.G. and O. Smithies. 1966. A simple hemagglutination system requiring small amounts of red cells and antibodies. Transfusion 6:67-73.
Wolfe, H.R. 1942. Precipitin production in chickens. I. Interfacial titers as affected by quantity of antigen injected and aging of antisera. J. Immunol. 44:135.
1 Department, Framingham State College, Framingham, MA, USA
2 Alltech Canada, Guelph, Ontario, Canada
3 Ohio State University, OARDC, Wooster, OH, USA








