SUMMARY
Bile acids are intended for use in animal feed as a novel feed additive to enhance fat absorption. As far as we know, few studies in avian species have been reported, and a safety study of bile acids has not been done. Therefore, a subchronic broiler chicken safety study was conducted to evaluate whether or not high-dose bile acids could affect the health of broiler chickens. A total of 324 1-day-old Arbor Acres broiler chickens were randomly assigned to 3 treatments with 6 replicates of 18 broiler chickens each. Broiler chickens were fed a corn-soybean meal diet supplemented with 0, 80 (the recommended available dose, RAD), and 400 mg/kg (5-fold of RAD) of bile acids for 42 d, respectively. Throughout the study, clinical observation and growth performance were measured. At the end of the study, broiler chickens were subjected to a full postmortem examination: blood samples were collected for clinical pathology, selected organs were weighed, and specified tissues were taken for subsequent histopathological examination. No treatment-related changes considered to be of toxicological significance were observed. Therefore, a nominal bile acids inclusion of 400 mg/kg was considered to be the no-observed adverse-effect level.
Key words: broiler chickens, bile acids, safety evaluation, blood parameters, organ development
2018 J. Appl. Poult. Res. 27:532–539 http://dx.doi.org/10.3382/japr/pfy040
DESCRIPTION OF PROBLEM
Bile acids (BAs) are natural components of human and animal bile [1]. They are derived from cholesterol in the liver [2] and further metabolized by bacteria in intestine. Bile acids are classified as primary or secondary. The primary BAs in human and broiler chickens are cholic acid (CA) and chenodeoxycholic acid (CDCA). However, CDCA and α-hyodeoxycholic acid (αHDCA) are the primary BAs in pigs [2]. In liver, BAs are conjugated with taurine or glycine and are secreted into the duodenum as conjugated BAs. In intestine, bacterial enzymes act on the primary BAs and convert them to secondary BAs by deconjugation, dehydroxylation, epimerization, and oxidation of BAs. Cholic acid is converted to deoxycholic acid (DCA) and CDCA to lithocholic acid (LCA). The enterohepatic circulation of BAs is the recycling of BAs between small intestine and liver, which is physiologically important. Although bile salt and BA absorption is efficient, some salts and acids are nonetheless lost with every cycle of the enterohepatic circulation. For example, about 500 mg of BAs are lost daily in humans [2].
Bile acids play an important role in the intestinal absorption of lipid [3]. Previous studies have found that secretion of bile is limiting in young broiler chickens, especially during the first week of life, resulting in low-fat digestion [4]. Young broiler chickens are unable to replenish BAs like older broiler chickens, and the decreased pool size of BAs may also contribute to the malabsorption of fat [5]. For this reason, BAs and its derivatives have been used in diets for young broiler chickens to improve fat digestion and absorption [6–8]. As shown in our previous study, dietary supplementation of porcine BAs in broiler chickens diets can effectively improve the growth performance, and carcass characteristics, as well as modulate the activity of intestinal enzyme lipoprotein lipase and lipase in the duodenum. The results indicate that BAs have the potential to improve absorption of dietary fat, carcass characteristics, and growth performance of broiler chickens [9].
However, BAs have been reported to induce cell injury and affect many mechanisms, such as damage to the plasma membrane, oxidative stress, apoptosis, and inflammation [10, 11]. As far as we known, there are a few conventional sub-chronic and chronic safety studies for BAs in livestock, and the effects of BAs have not been evaluated in broiler chickens yet. Therefore, as part of an extensive program of safety evaluation studies, a 42-d feeding experiment in broiler chickens was conducted to examine if the high-dose BAs could affect the physical health of broilers, including growth performance, organ index, clinical blood metabolites and hematology, and tissue pathological observation of broiler chickens. The high dosage was chosen to provide significant exposure to the test material, and the other dosages were set to provide data adequate for risk assessment purposes.
MATERIALS AND METHODS
Study Design
A total of 324 1-day-old Arbor Acres (AA) male broiler chickens [12] (average body weight, 44.04 g) were randomly assigned to 3 groups with 6 replicates of 18 broiler chickens each (108 broiler chickens per group). The broiler chickens were fed a corn-soybean basal diet supplemented with 0, 80 (the recommended available dose, RAD), and 400 (5-fold of RAD) mg/kg of BAs [13] for 42 d, respectively. Bile acid supplementation of 80 mg/kg is regarded as the recommended available dose according to the study of effect of BAs on broiler chickens conducted in our laboratory [9]. Song et al. [14] provided guidance for choosing appropriate feeding concentrations of BAs in mice by feeding mice CA, CDCA, DCA, LCA, and ursodeoxycholic acid (UDCA) at concentrations of 0.01, 0.03, 0.1, 0.3, 1.0, or 3% in each diet for 7 d. According to the results of body weight of mice, relative liver/body weight, serum alanine aminotransferase activity, and BA concentrations, the lowest concentration of each BA in the feed that causes hepatotoxicity in mice is CA and CDCA at 0.3%, DCA at 0.1%, and LCA at 0.03%. Thus, the 10-fold of the recommended available dose was supposed to be toxic to animals. Based on these findings, 400 mg/kg of BAs was added to the basal diet to investigate the effects of high dose BAs on growth performance, clinical blood metabolites, and organ development of broiler chickens. Clinical observations, body weight, and feed consumption were measured, and at the end of the scheduled period, the broiler chickens were killed and subjected to a full postmortem examination. Blood samples were taken for clinical pathology, selected organs were weighed, and specified tissues were taken for subsequent histological examination.
Broiler Type, Diet, and Management
The Animal Welfare Committee of China Agricultural University (Beijing, China) approved the Animal Care Protocol used in this experiment. All broiler chickens were raised in wire-floored cages in an environmentally controlled room with continuous lighting and had ad libitum access to feed and water throughout the experiment. The room temperature was maintained at 35?C for the first 3 d, after which the temperature was gradually reduced by 3?C a week until it reached 24?C and then maintained at this temperature until the end of the 42-d experiment. All broiler chickens were inoculated with inactivated Newcastle disease vaccine on day 7 and 21 and inactivated infectious bursal disease vaccine on day 14 and 28.
Broiler chickens were fed starter diets from day 1 to 21 and growth diets from day 22 to 42. Diets were fed in mash form. All nutrients contained in the basal diet met or exceeded the requirements suggested by the NRC [15]. The ingredients and chemical composition of the basal diet are shown in Table 1.
Sample Collection and Analytical Determination
Observations Cage-side observations, which included recording any changes in clinical condition or behavior, were made at least twice daily throughout the study. Growth Performance Broiler chickens body weight and feed consumption of broilers in each pen were recorded on day 21 and 42 after fasting for 12 h. The data were used to calculate average daily gain (ADG), average daily feed intake (ADFI), and feed conversion ratio (F: G) in each period and the whole period. Mortality was recorded daily to adjust feed consumption. Clinical Blood Parameters On day 21 and d 42, 1 broiler chicken that visually approximated the average weight from each pen was selected for blood sampling. The blood samples for hematology and clinical chemistry were collected by cardiac puncture: 1.5 mL of blood was collected in EDTA for hematology, whereas 2.5 mL of blood was collected for blood clinical chemistry analysis. The following hematological parameters were measured on samples collected using an analyzer: white blood cell, red blood cell, hemoglobin, mean corpuscular volume, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, red blood cell distribution width, platelet count, mean platelet volume, platelet distribution width, and plateletcrit. Clinical chemistry measurements were made on the serum collected from blood samples centrifuged at 3,000 × g for 10 min at room temperature, including total bilirubin (TBILI), total protein (TPRO), albumin (ALB), alkaline phosphatase (ALP), glucose (GLU), urea nitrogen (UN), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and creatinine (CRE). All metabolites were measured using a biochemical analyzer. These metabolites were included to cover a wide range of possible toxicities, to include possible effects on electrolyte balance, metabolism (carbohydrates, protein, fat, and minerals), and damage to the major organ systems.
Necropsy At the end of this experiment, those broiler chickens (n = 18) were killed by being bled from the jugular vein and decapitated after blood sampling on day 42. Then they were subjected to a full postmortem examination. Descriptions of all macroscopic abnormalities of broiler chickens killed and died during the experiment were recorded. At necropsy, the heart, liver, spleen, bursa of Fabricius, and thymus were weighed. Relative organ weight was expressed as the ratio to body mass (g/kg).
HistopathologyAnalysis A 1 cm piece of each small intestinal segment was taken in the middle and fixed in 10% buffered formalin for histological analysis. The liver and kidney were also cut into slices at a thickness of 1 cm and collected. The tissue samples were fixed in 10% buffered formalin for 48 h, and then treated following dehydration, clearing and paraffin embedding procedures. Paraffin sections of 5 μm thickness were stained with hematoxylin and eosin.
Statistical Analysis All data were analyzed using SAS 8.0 1-way ANOVA. A Duncan’s multiple comparison test was used to separate difference means among treatments. Data were assumed to be statistically significant when P < 0.05.
RESULTS AND DISCUSSION
Mortality and Observations
No treatment-related adverse clinical signs were observed. Mortality among treatments had no difference during the study (Table 2). Diets containing BAs were tolerated well and did not produce any general organ or systemic toxicity when fed to broiler chickens at doses as high as 400 mg/kg of the diet for a period of 42 d.
Growth Performance
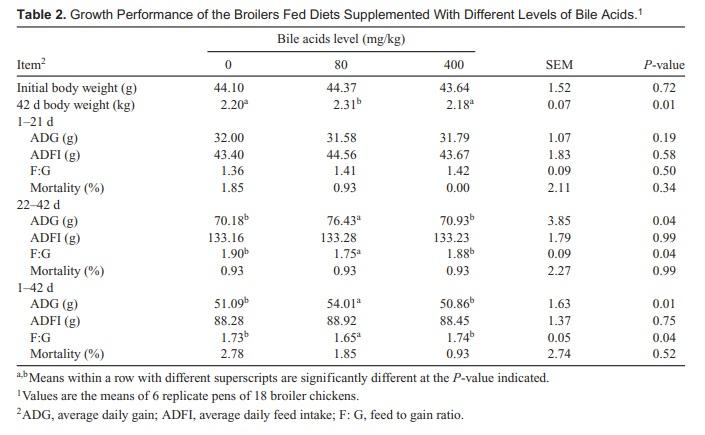
The effect of BAs on growth performance of broiler chickens is shown in Table 2. Broilers fed diets supplementing with 80 mg/kg BAs had a higher 42-d body weight (P < 0.01). No statistically significant differences were found in the starter phase (day 1–21) between treatments. In the grower phase (day 22–42) and during the overall experiment, ADG and F: G of broiler chicks in 80 mg/kg BAs treatment groups were higher than the control and 400 mg/kg BAs treatment (P < 0.01). Broilers fed diets supplementing with 400 mg/kg BAs had a similar growth performance to the control.
Clinical Blood Parameters
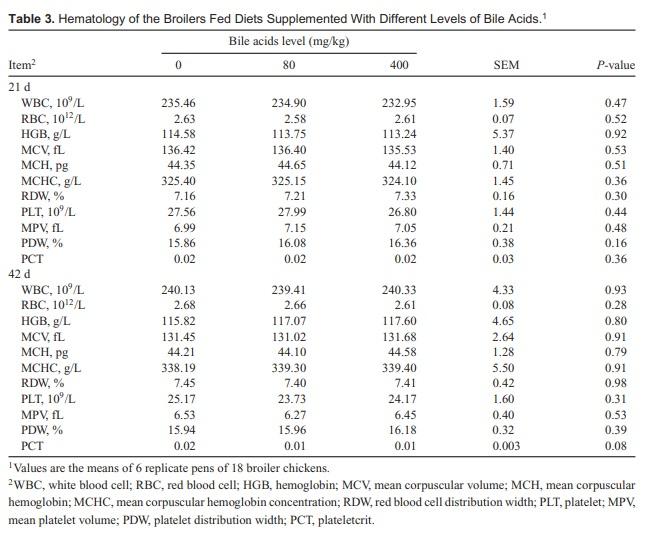
There were no significant differences in the hematological parameters among treatments (Table 3). As shown in Table 4, serum ALP, TPRO, ALB, UN, CRE, and TBILI concentrations were not affected by BAs inclusion. Compared with the control groups, broiler chickens fed 80 mg/kg BAs had a significant decrease in 21-d serum ALT and AST concentrations (P < 0.01), as well as serum AST concentrations in day 42 (P < 0.01). Serum ALT and AST had no difference between control groups and 400 mg/kg BAs treatment groups. Serum ALT and AST activity are traditional biochemical indices of liver function and are used clinically for diagnosis of liver injury. According to the decrease in serum ALT and AST concentration, the 400 mg/kg BAs supplementation in feed did not cause hepatotoxicity. The results suggested that high dosing BAs had no side effect on broiler chickens clinical blood parameters.
However, serum GLU levels in broiler chickens fed 400 mg/kg BAs were significantly lower than those fed control and 80 mg/kg BAs (P < 0.01). This finding indicated that BAs promoted glucose metabolism. Bile acids stimulate glucose uptake, which is commonly used as an energy source and a metabolic intermediate in the animal body. The improvement in the availability of glucose can also be found both in birds fed a synthetic emulsifier, glycerol polyrthylene glycol ricinoleate [16] and in broiler chickens fed a natural emulsifier, lysophospholipid [17]. No difference in the physical activity among 3 treatments was observed. Piekarski et al. have found that diets containing 0.5% CDCA were well tolerated and devoid of side effects [18].
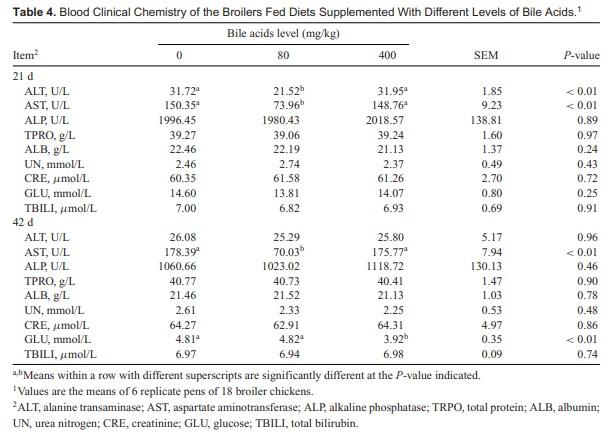
An important role for BAs as signaling molecules has emerged in recent years. Bile acids activate mitogen-activated protein kinase pathway, are ligands for the G-protein-coupled receptor TGR5 [19], and activate nuclear hormone receptors such as farnesoid X receptor α [20]. Through this mechanism, BAs regulate glucose metabolism, and they are closely related to glucose and lipid homeostasis. Therefore, BAs are implicated not only in the hepatic regulation of cholesterol metabolism but also in that of glucose [21]. The high-dose BAs feeding resulted in aa decrease in the serum glucose concentrations of 42-d-old broiler chickens. Piekarski et al. [18] also found a significant decrease in glucose of broiler chickens fed CDCA-supplemented diets. Zheng et al. [22] performed a subchronic toxicity test on rats by feeding BAs as feed additive at 0, 60, 600, and 3,000 mg/kg for 90 d, and no obvious harmful effects were observed among treatments. Moreover, the serum glucose concentration of rats in 600 mg/kg BAs treatment was decreased at 45 and 90 d. However, the potential glucose-lowering action of BAs in avian species has not been thoroughly described.
Necropsy and Histopathology
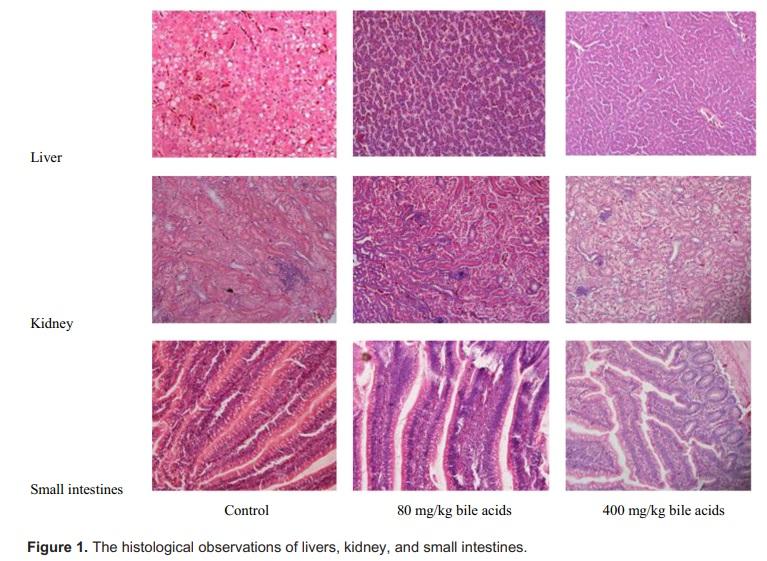
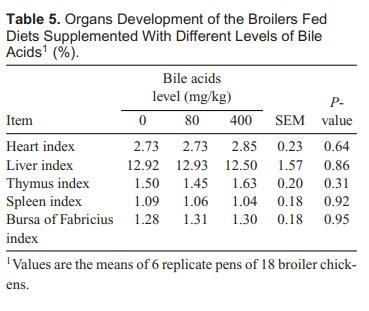
Analysis As shown in Table 5, no significant differences were observed in organ development among groups. At the end of the experiment, no significant changes were observed in histopathology analysis of those tissues compared to the control broiler chickens. Figure 1 shows the histological observations of livers, kidney, and small intestines. No histological changes were considered to be related to treatment.
These doses did not produce any negative effects on performance, hematology, or cause any treatment-related clinical signs. Consumption of BAs up to 400 mg/kg appeared to have no side effects, defined as reported adverse events or undesirable changes in clinical chemical parameters, hematological parameters, and postmortem examination.
CONCLUSIONS AND APPLICATIONS
1. The results indicated that long-term use of high-dose BAs (5-fold of the recommended available dose) could not induce any treatment-related changes that were considered to be of toxicological significance. Therefore, a nominal BAs concentration of 400 mg/kg was suggested to be the no-observed-adverse-effect level following daily administration to broiler chickens for 42 d.
2. BW and ADG were improved by 80 mg/kg BAs inclusion; therefore, adding optimal dosage of BAs could improve broiler chicken performance.
3. The significant decrease in the glucose concentrations at the end of the trial period suggested that high dosage of BAs had a potential glucose-lowering action and promoted glucose metabolism consequently