Experience in monitoring IBD antibody titers of broiler birds of two close house farms involving three IBD vaccinations per cycle
Published: March 19, 2015
By: Christopher Hettiarachchi ,Managing Director (Industrial Microbiologist) M.Sc.(Ind.Mcb.), PGD (Ind.Mcb.), B.Sc.(Biol)
Back ground
One of the challenges I had during my work as the research and development manager of one of the leading semi-integrated (rearing/processing) broiler operations in Sri Lanka was to investigate why infectious bursal disease (IBD) out breaks occurred from time to time in the two close house broiler farms belonging to the company despites of administering three IBD vaccines during a production cycle. It was decided to approach the problem in two fronts; monitoring of antibodies against IBD vaccines during one cycle and to find out effectiveness of vaccination protocols. This report is on the first of the two; monitoring of antibodies against IBD vaccines of one cycle in two cages.
IBD or Gumboro disease, caused by an avibirnavirus, has been an economically significant, widely distributed condition affecting immature chickens. Both, classical and virulent strains of the virus cause severe economical losses in broilers in addition to other affects such as immune-suppression, growth retardation and liveability and productivity lost. A comprehensive bio-security system and a good vaccination program is necessary to control this disease and reduce production loses.
The two farms in question are located far away from each on the poultry belt of Sri Lanka. The farm in which the trial was carried out consists of 6 close houses each measuring 360 feet by 40 feet. Each house accommodates 32,000 birds (about 0.46 ft2 per bird till the first harvest at day 27-28). Average production statistics of the farm are; mortality - 2.6-2-9%, feed conversion ratio (FCR) – 1.68-1.78, weight at day 28 – 1.6kg. The number of cycles per year has been 6.5 and all in all out rearing system is practiced. Cleaning and disinfection program is closely monitored and bacterial counts on floor and other related surfaces are also monitored after disinfection and the bacterial counts are maintained close to zero. A strict bio-security program is in place to avoid contamination. IBD vaccination program consisted of day 12 with IBD Bur 706 ( live intermediate), day 17 and day 24 with IBD AviproLC75 (live intermediate). The development of antibodies against IBD vaccines was monitored during one production cycle of two cages (of the 6 cages) at one farm (of the two). Serological monitoring using ELISA (Enzyme Link Immunosorbent Assay) technology is an accepted method of monitoring antibody levels and IBD IDEXX ELISA (USA) test kits were used in this monitoring.
Method
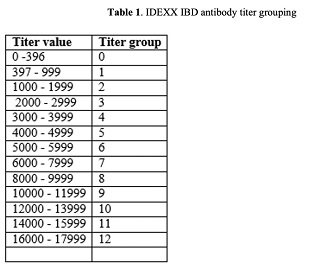
In-put of chicks for the trial were from two different hatcheries; hatchery I and II. Chicks from hatchery I numbering 11,500 were placed in on the 19th of December, 2011 and the remaining 20,500 chicks from hatchery II were placed on the 20th of January 2011. Twenty chicks from each strain were sacrificed and serum samples were separated for day 1 sampling and thereafter, 20-24 chicks were randomly selected for bleeding which was done at day 12, day 19, day 24 and day 29 (at slaughter). Serum samples were subjected to analysis for antibodies against IBD using IDEXX IBD test kit (USA). Titer groups are categorized according to IDEXX Xchek protocols and the titer values and their respective titer groups are given in table 1. The plates were read using microwell plate reader Biotek, model ELX800IU.
Table 1. IDEXX IBD antibody titer grouping
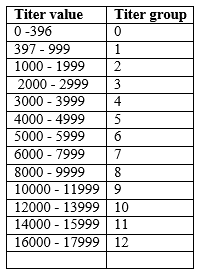
Percentage frequency distribution of each titer group for each bleeding were calculated, a graph was plotted and is shown in Fig.1. The change of mean titers against ABD vaccination over the production cycle of the trial is shown in Fig. 2. The results of IBD antibody monitoring of cage 2 of farm 1 are also given in table 2 for comparison. Relevant production statistics of IBD affected batch of farm 2 where same vaccination protocols are done are also given in table 3 for better understanding of the progress of the disease.
Fig. 1. Frequency distribution of antibody titer groups against IBD vaccines at different ages of birds (using IDEXX IBD test kits) of cage 1 of farm 1
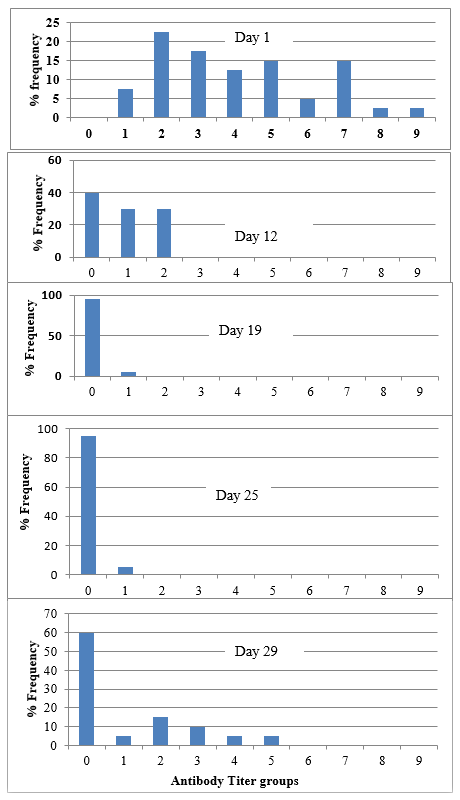
Fig. 2. Change of mean antibody titers against IBD vaccination over the production cycle of the trial of cage 1 of farm 1
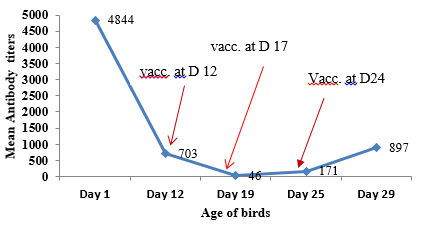
Table 2. Mean IBD antibody titer levels and % coefficient of variance of broiler birds of cages 1 & 2 studied at farm 1
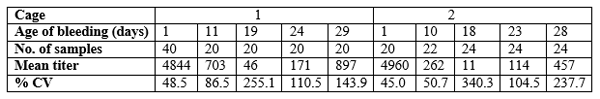
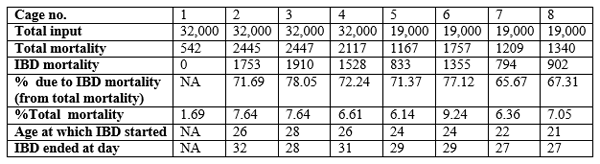
Table 3. Pattern of IBD mortality of farm 2 where same vaccination protocols have been carried out
Results and Discussion
Immediately after second vaccination, antibody titer levels have dropped drastically from a mean titer of 4844 ( maternally derived antibody) to as low as 44 at day 19 making all the birds vulnerable to IBD field challenge and this remains below the positive cut of point until day 24 . Similar trend was observed in the change of antibody levels over time in cage 2 as well where same IBD vaccination protocol is followed (Table 2, cage 1 & 2 and Fig.2).
Susceptibility of the birds to field challenge even at day 26 is evidence when the mortality pattern of IBD affected batch at farm 2 is considered (table 3). Birds in cage 2,3 and 4 must have exposed to IBD virus 2 to 3 days earlier before mortality started and the age of those birds could fall within the time period the birds having very low level of antibody titers (Table 3). These results suggest that IBD vaccination protocols (at day 12, 17 and 24) carried out at the two farm sites do not protect the broiler birds sufficiently against IBD field challenges.
Following images show some of the strict management practices that are carried out at the farm to avoid pathogenic microorganisms getting from one batch to the next.
Cleaning and disinfection of the cages

Environmental monitoring of surfaces for microbial counts using contact slides after disinfection to reduce chances of transfer of pathogens from previous batch to the subsequent batch

Proper brooding practices carried out at the farm

ELISA equipment set up for carrying out antibody monitoring

Please note:
By increasing number of samples could have improved the results.
Related topics:
Authors:
Recommend
Comment
Share
5 de agosto de 2023
Dear Dr,
With another disease, as ND, APV, IBV....how can we know Titer Grouping as IBD above - examples: Group 0 titer from 0 to 396, Group 1 titer from 397 to 999......( using IDEXX Test KIT)
Best regards,
Recommend
Reply
INTA Argentina
19 de septiembre de 2016
Hello Mr. Hettiarachchi.
I'm interested about the IDEXX score grouping you have made (Table 1). I read that it's made according to IDEXX Xcheck protocols, and I would like to know were to find these protocols and/or some references to understand how that categorical scale is constructed or what does it mean for a chicken to be within a certain category.
I use IDEXX IBD kit for research purposes and it would be very helpful to know what is that scale based in.
If you can provide any information regarding this, I would be very grateful.
Kind regards.
Matías Richetta
PhD Student
Recommend
Reply

Would you like to discuss another topic? Create a new post to engage with experts in the community.









.jpg&w=3840&q=75)