
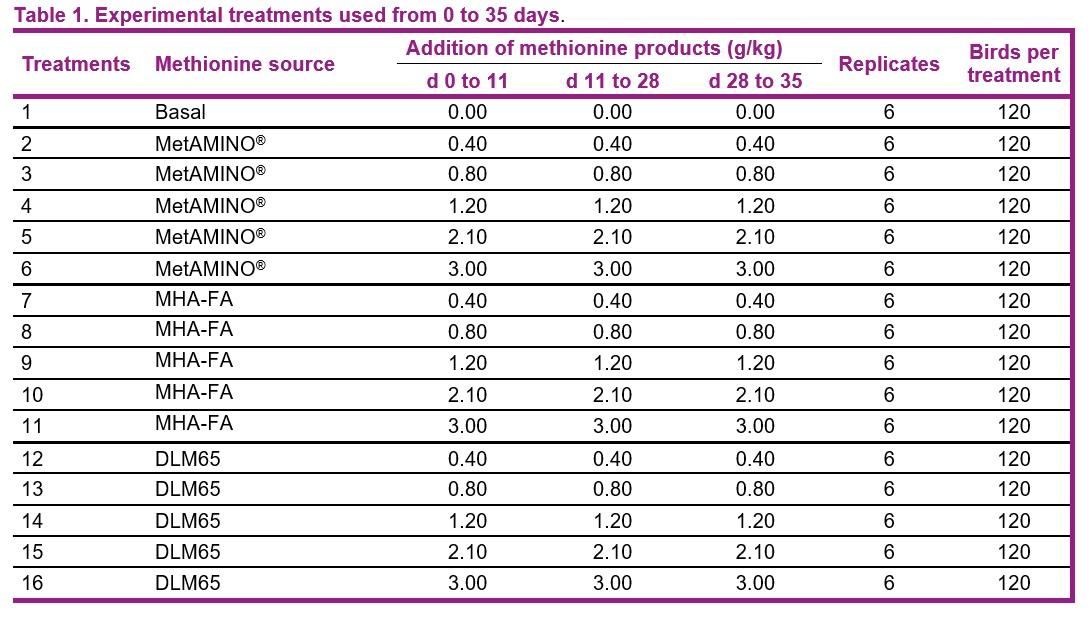
Broiler trial in the Netherlands confirms 2018 EFSA scientific opinion on methionine sources while validating the experimental approach
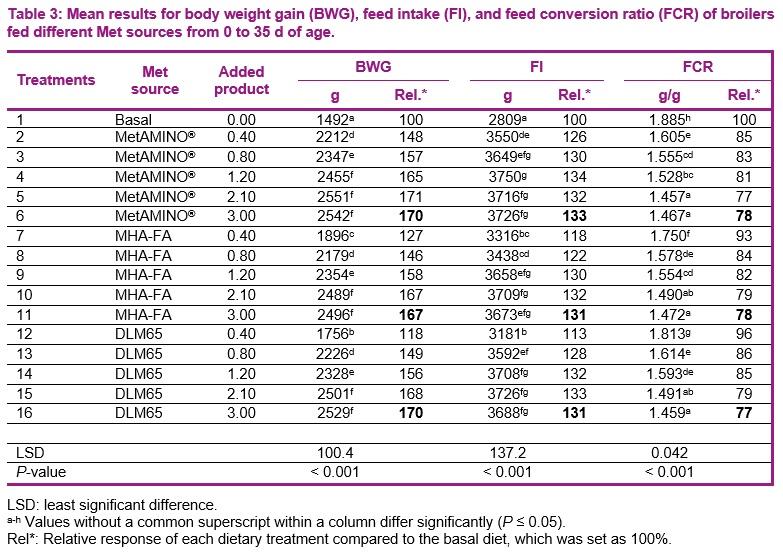
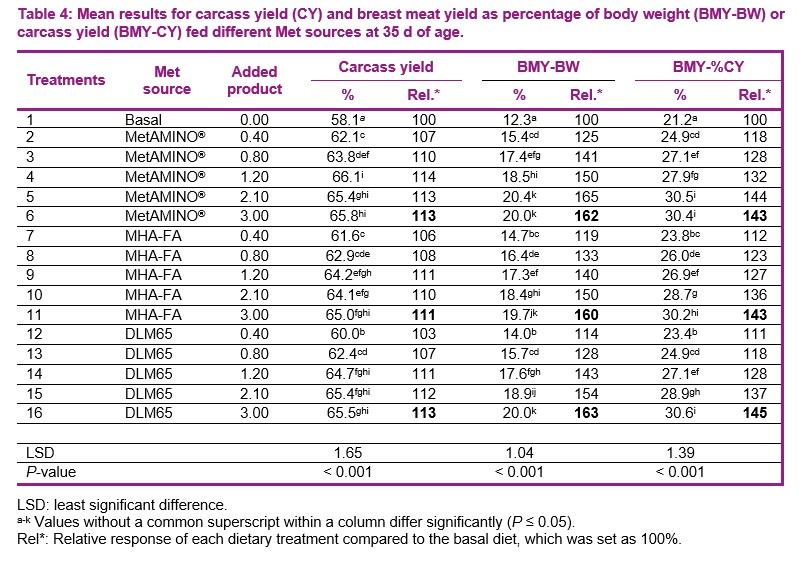
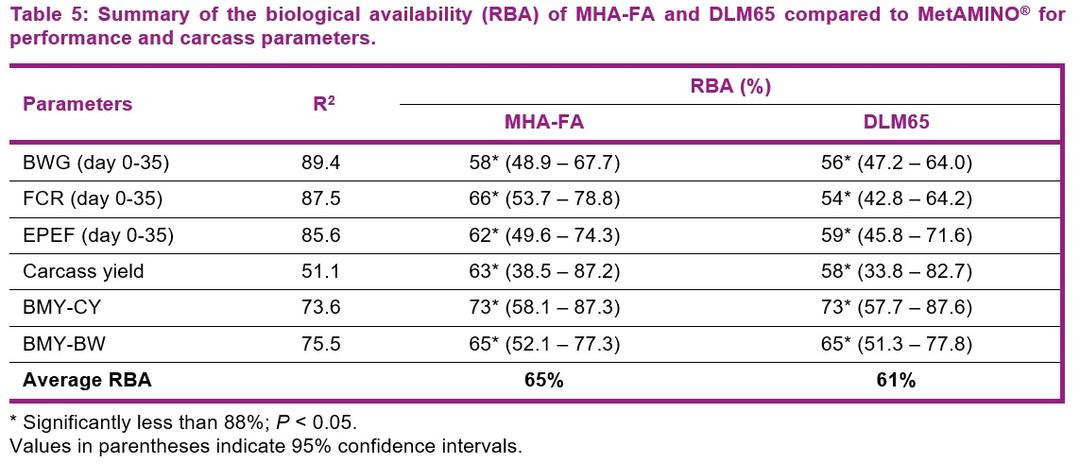
- The present study conducted at Schothorst Feed Research resulted in an average bioavailability of MHA-FA and diluted DLM65 of 65% and 61%, respectively compared to MetAMINO® considering the overall growth and carcass parameters.
- These results demonstrate that the relative bioavailability of MHA-FA is significantly lower than its active content of 88% and consistent with the 2018 EFSA scientific opinion of 66% on product basis (75% on equimolar basis).
- The average RBA of 61% for DLM65 was close to the expected value of 65% (because of dilution to 65% purity) and thus confirming the appropriateness of using simultaneous dose-response approach to determine the RBA of nutrient sources.
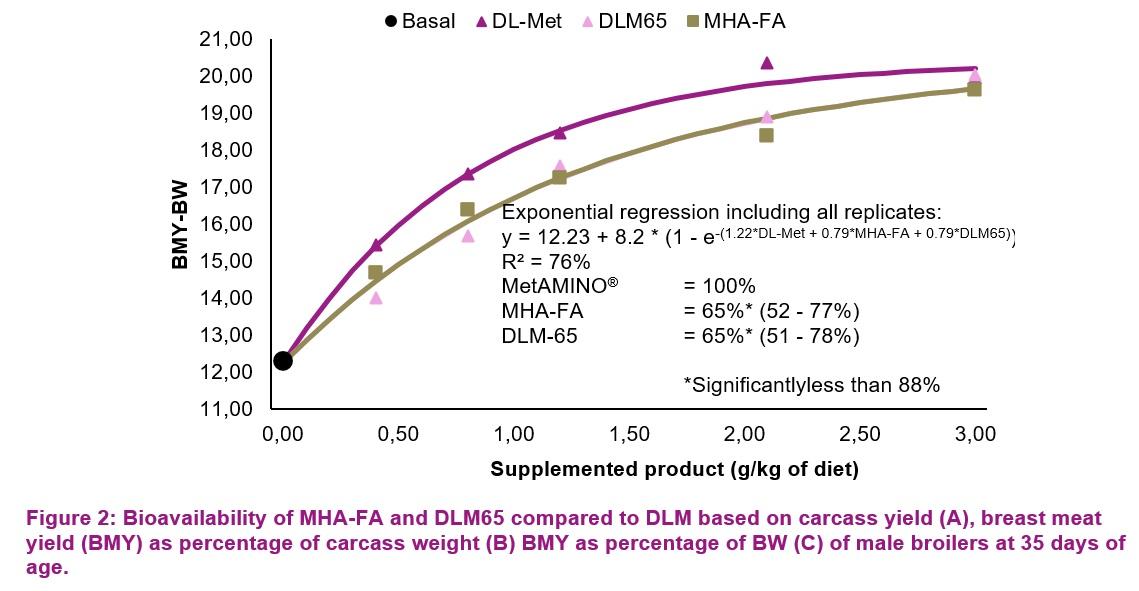
 The dig. Met+Cys levels of the starter, grower, and finisher basal diets were 0.62, 0.54 and 0.51%, respectively (Table 2). Growth performance parameters were evaluated for each phase. On day 35, two birds per pen which were close to the average BW of the pen, were selected for carcass evaluation and individually weighed. The carcasses were chilled for 4 hours before cutting up. Carcass weight (kg) was defined as plucked, bled and eviscerated carcasses without head, neck and feet. Carcass yield (CY) was expressed as % of BW at day 35. Breast meat weight included both musculus pectoralis major and minor (without skin). Breast meat yield was expressed as % of BW at day 35 (BMY-BW) and also as % of the carcass weight (BMY-CW). Growth performance and carcass data were subjected to multi-exponential regression analysis using the nonlinear-regression procedure described by Littell et al. (1997).
The dig. Met+Cys levels of the starter, grower, and finisher basal diets were 0.62, 0.54 and 0.51%, respectively (Table 2). Growth performance parameters were evaluated for each phase. On day 35, two birds per pen which were close to the average BW of the pen, were selected for carcass evaluation and individually weighed. The carcasses were chilled for 4 hours before cutting up. Carcass weight (kg) was defined as plucked, bled and eviscerated carcasses without head, neck and feet. Carcass yield (CY) was expressed as % of BW at day 35. Breast meat weight included both musculus pectoralis major and minor (without skin). Breast meat yield was expressed as % of BW at day 35 (BMY-BW) and also as % of the carcass weight (BMY-CW). Growth performance and carcass data were subjected to multi-exponential regression analysis using the nonlinear-regression procedure described by Littell et al. (1997).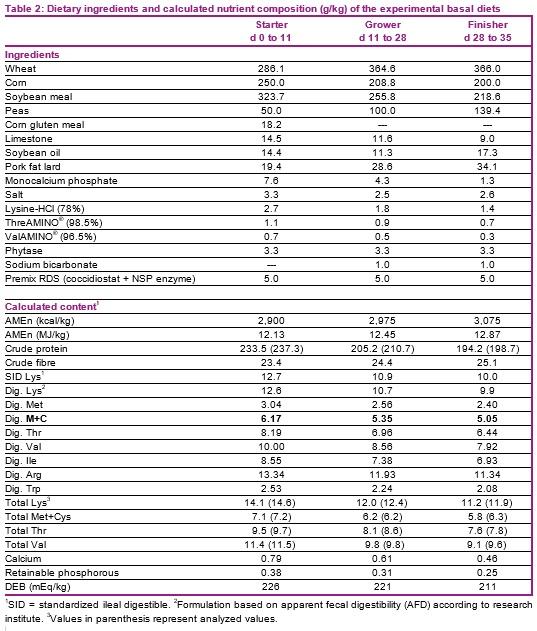
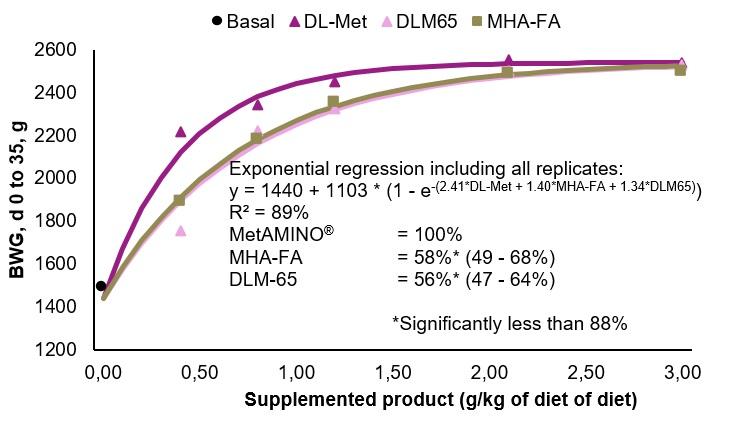
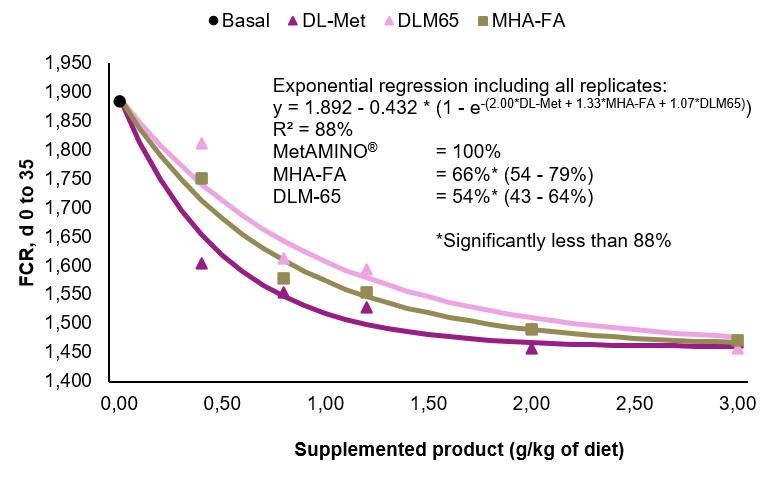
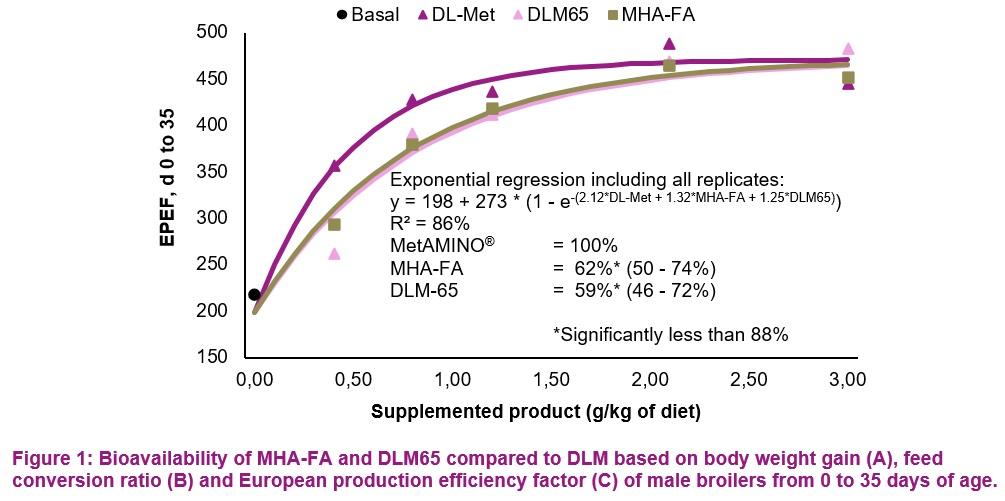
A)
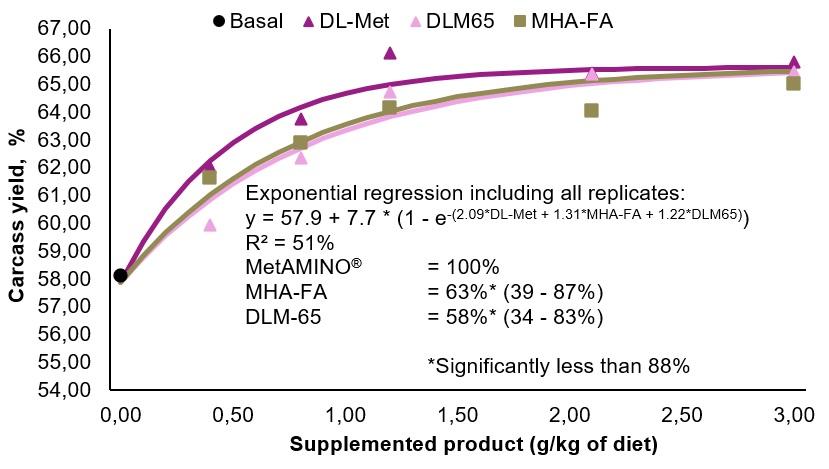
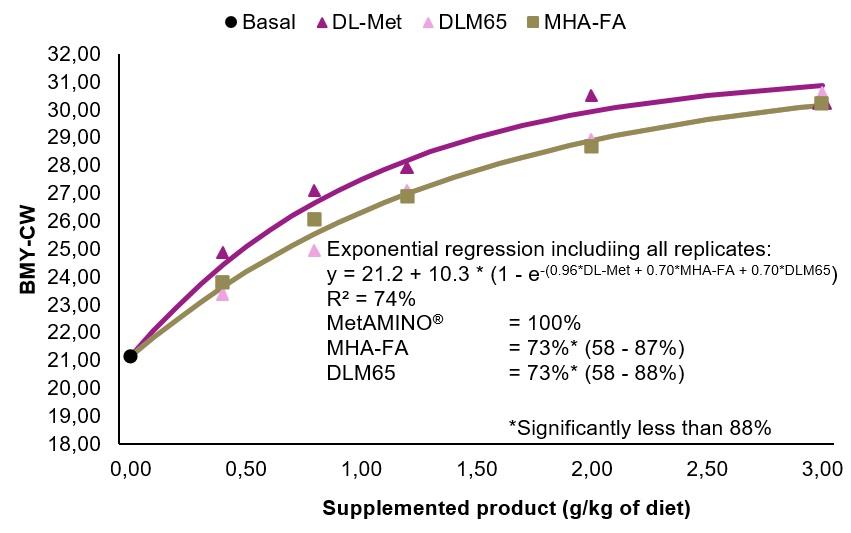
C)
European Food Safety Authority (EFSA), 2018: Safety and efficacy of hydroxy analogue of methionine and its calcium salt (ADRY+®) for all animal species, EFSA Journal 16(3): 5198 .
Jansman, A.J.M., C.A. Kan, and J. Wiegenga, 2003: Comparison of the biological efficacy of DL-methionine and hydroxy-4-methylthiobutanoic acid (HMB) in pigs and poultry, CVB documentation report nr. 29, Centraal Veevoederbureau (CVB, Lelystad, The Netherlands.
Hoehler, D., A. Lemme, K. Roberson and K. Turner, 2005a: Impact of methionine sources on performance in turkeys, Journal of Applied Poultry Research 14: 296-305.
Hoehler, D., A. Lemme, S.K. Jensen, and S.L. Vieira, 2005b: Relative effectiveness of methionine sources in diets for broiler chickens, Journal of Applied Poultry Research 14: 679-693.
Lemme, A., D. Hoehler, J.J. Brennan, and P.F. Mannion, 2002: Relative effectiveness of methionine hydroxy analog compared to DL-methionine in broiler chickens, Poultry Science 81: 838-845.
Lemme, A.. A. Helmbrecht, and S. Mack, 2012: Commercial methionine sources in poultry, AMINONews® Review: pp 39.
Littell, R.C., P.R. Henry, A.J. Lewis and C.B. Ammermann, 1997: Estimation of relative bioavailability of nutrients using SAS procedures, Journal of Animal Science 75: 2672-2683.
Sauer, N., K. Emrich, H.-P. Piepho, A. Lemme, M.S. Redshaw, and R. Mosenthin, 2008: Meta-analysis of the relative efficiency of methionine-hydroxy-analogue-free-acid compared with DL-methionine in broilers using nonlinear mixed models, Poultry Science 87: 2023-2031.
Sangali, C.P., Giusti Bruno L.D., Vianna Nunes, R., Oliveira Neto, A.R., Pozza, P.C., Oliveira, T.M., Frank, R. and Schone, R.A., 2014. Bioavailability of different methionine sources for growing broilers. Revista Brasileira de Zootecnia, 43: 140–145.
Vazquez-Anon, M., D. Kratzer, R. Gonzalez-Esquerra, I.G. Yi, and C.D. Knight, 2006: A multiple regression model approach to contrast the performance of 2-hydroxy-4-methylthio butanoic acid and DL-methionine supplementation tested in broiler experiments and reported in the literature, Poultry Science 85: 693-705.



Dear Ms. Peris, Thank you for your comments. The first objective of this trial was to determine the relative bioavailability of Met sources in broilers. As stated by Jansman et al., (2003) proper studies are those that include a basal diet clearly deficient in Met, and at least 3 levels of supplementation of the test products. As in any dose-response trial, it is crucial that enough data points are included to properly describe the different sections of the response curve. Thus, it is important that the basal diet (starting point) is clearly deficient in the nutrient of interest. In this study, the basal diet was clearly deficient in Met confirmed by the significant reduction in growth performance. The 5 increasing levels of each Met source improved growth performance following - as expected - the law of diminishing returns and with data points well distributed along the complete response curve (deficiency and above plateau). Therefore, all criteria for a proper bioavailability study were met. Accurate “bioavailability values” cannot be determined or extrapolated by only comparing nutrient sources above the requirement. Using this approach, data from this trial will suggest that at the highest Met inclusion level a diluted DL-Met to 65% purity (DLM65) is equally effective as DL-Met with 99% purity! Certainly, this is not correct. As these points are in the plateau section of the response curve (requirement already met), no further responses are expected above this level (law of diminishing returns) which does not allow for any proper comparison of nutrient sources. Therefore, to properly determine the bioavailability of nutrient sources, trials need to include a basal diet clearly deficient in the nutrient of study with enough data points representing the different sections of the response curve.
The second objective of this trial was to validate the mathematical suitability of the multi-exponential regression analysis for estimating the relative bioavailability. For this, we used diluted DL-Met to a purity of 65% (DLM65) from which its bioavailability is known a priori (close to 65%). As DLM65 was included, equimolar comparison would have not been possible in this experiment (same inclusion as DLM). However, proper experiments designed either on equimolar or product basis led to the same conclusion (Jansman et al., 2003; Hoehler et al., 2005, EFSA, 2018). For example, based on an extensive literature study Jansman et al. 2003 reported an average bioefficacy of liquid HMTBA of 77% on equimolar basis compared to DL-Met in broilers. This translates to a bioefficacy of 68% on product basis compared with DL-Met (0.77 * 88). Similarly, the European Food Safety Authority (EFSA, 2018, Appendix A) released a scientific opinion on liquid HMTBA and its calcium salt, confirming a bioavailability of 75% on equimolar basis which is equivalent to 66% (0.75 * 88) on product-to-product basis for liquid MHA-FA. These previous results are in agreement with those obtained in the current study which resulted in an average bioavailability of HMTBA (MHA-FA) and DLM65 of 65 and 61%, respectively. Therefore, proper studies conducted either on equimolar or product basis will lead to the same conclusion.


METAMINO®


METAMINO®



United States





