1. Introduction
Poultry is a food that has been highly appreciated by man since time immemorial. It is an important, low cost source of animal protein, rich in nutrients, phosphorus, other minerals, and B-complex vitamins (FAO 2010). Chicken carcasses have higher pathogenic and spoilage bacterial counts than most other foods, where carcass can be contaminated at several points throughout the processing operation during scalding, de-feathering and evisceration as well as cross contamination from other birds and processing equipment. Several indicators can be useful to evaluate hygiene level of meat such APC and total psychrotrophic counts. Total Enterobacteriaceae count and total coliforms count are more frequently used to assess enteric contamination and commonly used in slaughterhouses as indicators of faecal as well as environmental contamination (Gonzalez and Domingues, 2006). Moreover, total staphylococci count and Staphylococcus aureus counts, which are present on hand, mucous membrane and skin of man, birds and animals, are good indicators of poor personal hygiene, poor handling and temperature control (Rindhe et al., 2008). Contamination of poultry meat with foodborne pathogens remains an important public health issue, where many food poisoning bacteria contaminate chicken meat (Mbata, 2005). Therefore, the present study aimed to evaluate the bacteriological quality of some freshly slaughtered chicken carcasses through Determination of APC, Psychrotrophic count, Enterobacteriaceae count Coliform counts, total Staphylococci count, and isolation and identification of Salmonella, S. aureus, E. coli O157:H7, Listeria monocytogenes and Clostridium perfringens.
2. Material and methods
2.1. Collection of samples
A grand total of one hundred random samples of chicken carcasses (slaughtered, plucked and eviscerated) were collected from local commercial retail shops in BeniSuef city. The collected samples were kept in separate plastic bags, transferred directly to the laboratory in an insulated ice box under complete aseptic conditions without any delay to evaluate their bacteriological quality.
2.2. Preparation of samples (USDA, 2011)
Twenty five grams of the examined samples were removed by sterile scissors and forceps after surface sterilization by hot spatula, transferred to a sterile polyethylene bag, and 225 ml of 0.1 % sterile buffered peptone water were aseptically added to the content of the bag. Each sample was then homogenized in a blender at 2000 rpm for 1-2 minutes to provide a homogenate of 1/10 dilution. One ml from the original dilution was transferred with sterile pipette to another sterile test tube containing 9 ml of sterile buffered peptone water 0.1 % and mixed well to make the next dilution, from which further decimal serial dilutions were prepared. The prepared dilutions were subjected to the following examinations.
2.3. Determination of APC (USDA, 2011)
It was done using standard plate count agar media.
2.4. Determination of total psychrotrophic count (USDA, 2011)
It was done using standard plate count agar media.
2.5. Determination of Enterobacteriaceae count ( ISO, 2001)
It was done using violet red bile glucose agar media (VRBG).
2.6. Determination of total coliform count (FDA, 2002)
It was done using violet red bile agar media (VRB).
2.7. Determination of total Staphylococci and Staphylococcus aureus count (USDA, 2011)
It was done using Baird Parker agar media.
2.8. Isolation and Identification of Salmonella (FDA, 2011a)
It was done using Rappaport Vassilidis broth and Xylose Lysine Desoxycholate (XLD) agar.
2.9. Isolation and Identification of E. coli O157:H7 (FDA, 2011b)
It was done using E.coli broth supplemented with novobiocin and sorbitol MacConkey agar media.
2.10. Isolation and Identification of Clostridium perfringens (FDA, 2001)
It was done using cooked meat medium and Tryptose-sulfite-cycloserine (TSC) agar containing egg yolk emulsion.
2.11. Isolation and Identification of Listeria monocytogenes (Hitchins, 2003)
It was done using buffered Listeria enrichment broth and Oxford agar media.
3. Results
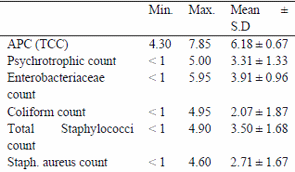
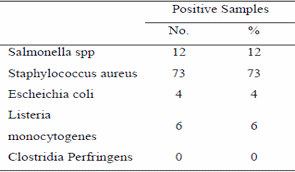
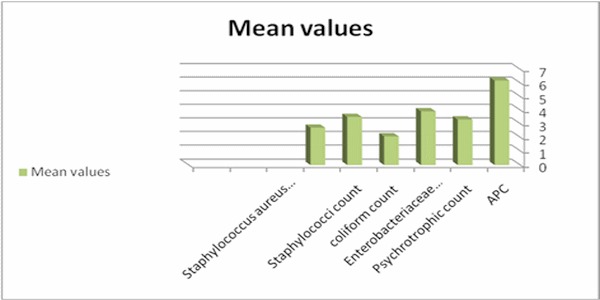
Table (1) and Fig.(1) reported that APC (log cfu/g) in the examined samples varied from 4.30 to 7.85 with a mean value of 6.18 ± 0.67. The total psychrotrophic count varied from <1 to 5.00 with an average value of 3.31 ± 1.33 , the total coliforms varied from <1 to 4.95 with an average value of 2.07 ± 1.87, the total staphylococci count varied from <1 to 4.90 with a mean value of 3.50 ± 1.68 and S. aureus count varied from 0 to 4.60 with an average value of 2.71 ± 1.67. Results given in table (2) showed that the incidences of isolated Salmonellae, St.aureus, E. coli and listeria monocytogenes were 12, 73, 4 and 6 %.
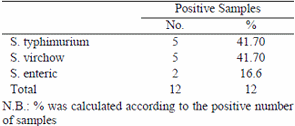
Clostridium perfringens failed to be detected in the examined samples. Regarding the results in table (3) the incidence of pathogenic E. coli serotypes isolated from the examined chicken carcasses were E. coli O55 (50%) and E. coli O86A (50%). Table (4) reported that Salmonellae could be identified serologically as S.typhimurium (41.70%), S. virchow (41.70%) and S. enteric (16.60%).
Fig. (1): Mean values of bacterial counts (log10cfu/g) in the examined chicken carcasses
4. Discussion
Microbial contamination of poultry carcasses is a natural result of different procedures necessary to produce retailed products from living birds. Most of bacterial contaminants are non pathogenic; however, poultry are known to harbour a large number of bacteria that are pathogenic to human being (Zhang et al., 2001). Several indicators can be useful to evaluate hygiene levels during meat slaughtering process. Aerobic plate count (APC) is commonly used to evaluate the hygiene of the entire meat production process. Nearly similar results were reported by Santosh Kumar et al. (2012) (6.23 log cfu/g), Sengupta et al.(2012) (6.39 log cfu/g) and Omorodion and Odu (2014) (5.96 log cfu/g) .On the other hand, higher counts were reported by Barbuddhe et al. (2003) (7.34 log cfu/g), Huong et al. (2009) (11.1 logcfu/g) and Bhandari et al. (2013) (7.24 log cfu/g). Lower counts were reported by Rindhe et al. (2008) (3.67 log cfu/g), Chaudhry et al. (2011) (5.07 log cfu/g), high numbers is a good indication of poor hygiene and poor temperature control. Higher percentage of Salmonella were reported by Abdellah et al. (2009) (57%), Huong et. al. (2009) (62.79%), Bhandari et al. (2013) (46.2%) and Lertworapreecha et. al. (2013) (67.5%). Moreover, Joshi and Joshi (2010) reported that Salmonella was isolated from all examined chicken carcasses (100%). On contrary, Salmonella was isolated in low percentage from chicken carcasses as reported by Cohen et al. (2007) (1.6%), Abdellah et al.(2008) (2.08%) , Colmegna et al. (2009) (1.1%) and Kozacins et al. (2012) (7.46%) . On the other hand, Salmonella could not be isolated from chicken carcasses as reported byVaidya et al. (2005) , Lindblad et al.(2006) , Selvan et al. (2007), Shaltout, F.A.(2009) and Javadi and Safarmashaei (2011). Presence of salmonellae in chicken meat may be attributed to the healthy state of the living bird which carries salmonellae, bad hygienic conditions during slaughtering, cross contamination either from other birds, instruments, machines, workers, scalding tanks, defeathering machines, crop removal, manual evisceration , during slaughter, intestinal contents can spill and contaminate the muscle and organs of the chicken, which is the important source of presence of Salmonella in meat and chilling tanks (Paiao et al., 2013). E.coli O157:H7 failed to be detected in the examined samples. Such results agree with results reported by Baran and Gulmez (2000) , Joa et al.(2004),Hajian et al. (2012) and Kalin et al.(2012). On the other hand, E.coli O157:H7 was isolated in low percents byAkkaa et al. (2006) (1.05%) and Kiranmayi and Krishnaiah (2010) (2%). On contrary, higher results were obtained by Chang et al. (2013) (40%). Although E.coli0157:H7 is mostly found in ruminant animal and it is occasionally associated with other livestock and various foods of animal origin, experience suggests that it is rare in poultry ,whether in the live birds or on processed products (Mbata 2005) .
Similar prevalence of L. monocytogenes was reported Kozacinski et al. (2006) (3%), Colmegna et al. (2009) (3%) and Kozacins et al. (2012) (4.5 %). Lower percentages were reported by Molla et al. (2004) (1.9%) and Cohen et al. (2007) (0.5%). L. monocytogenes is an important foodborne pathogen, which has been isolated from many natural environments, such as water, soil, sewage, mud, gut of poultry and feces Yeh (2004), Donnelly et al. (1992) and Nickelson and Finne, 1992). It is considered an environmental contaminant, therefore, cross contamination easily occurs in traditional shops during preparation of chicken carcasses ready to be sold.
Clostridium perfringens (C.perfringenes) failed to be isolated from examined samples. Such results agrees with Shaltout (2009). On Contrary, C. perfringens was isolated in higher percentages by Hall and Angelotti (1965) (58%), Miwa et al. (1998) (84%), Singh et al. (2005) (70.4%) and Nowell et. al. (2010) (66%). On the other hand, other studies could detect C.perfringens in lower percentages as Craven et al. (2003) (4%), Cohen et al. (2007) (7.2%) and Thangamani and Subramanian (2012) (3.81%).The organism is an obligate anaerobe that is relatively tolerant to oxygen and can be found in low numbers in the alimentary tract of poultry. When present in meat, growth is favoured by conditions in which oxygen has been dispelled. However, growth of the organisms cannot occur if the meat is held below 15OC, the problem is easily avoided by refrigerated storage. Regarding all the discussed results, chicken carcasses could be contaminated in all stages of production starting from the live bird carrying microorganisms during transportation to the slaughter house, during stages of slaughtering, bleeding, scalding, defeathering, evisceration, washing and storage. Therefore, good hygienic practices should be followed in every step of processing.
This article was originally published in Benha Veterinary Medical Journal, VOL. 28, NO. 2:74-82, 2015.
5. References
Abdellah, C., Fouzia, R. F., Abdelkader, C., Rachida, S. B., Mouloud, Z. 2008. Occurrence of Salmonella in Chicken Carcasses and Giblets in MeknèsMorocco. Pakistan Journal of Nutrition, 7(2):231-233, 200.
Abdellah, C., Fouzia, R. F., Abdelkader, C., Rachida, S. B., Mouloud, Z. 2009. Prevalence and anti-microbial susceptibility of Salmonella isolates from chicken carcasses and giblets in Meknès, Morocco. African Journal of Microbiology Research 3(5):215-21.
Akkaya, L., Atabay, H.I., Kenar, B., Alisarli, M. 2006. Prevalence of verocytotoxigenic Escherichia coli O157:H7 on chicken carcasses sold in Turkey. Bull Vet InstPulawy 50:513-516.
Al-Jasser, M.S. 2012. Effect of cooling and freezing temperatures on microbial and chemical properties of chicken meat during storage. Journal of Food, Agriculture & Environment. 10(1):113- 116.
Barbuddhe, S.B., Swain, B.K., Chakurkar, E. B., Sundaram, R.N.S. 2003. Microbial quality of poultry meat with special reference to Listeria monocytogenes. Indian Journal of Poultry Science. 38(3): 305- 307
Baran, Fatma, Gulmez, M. 2002. The Occurence of Escherichia coli O157:H7 in the Ground Beef and Chicken Drumsticks. Internet Journal of Food Safety 2:13-15.
Barnes, E.M. 1976. Microbiological problems of poultry at refrigerator temperature. A review. Journal of Scientific Food Agriculture, 27:777.
Bhaduri, S., Cottrell, B. 2001. Sample preparation methods for PCR detection of Escherichia coli O¬157:H7, Salmonella typhimurium and Listeria monocytogenes on beef chuck shoulder using a single enrichment medium. Molecular and Cellular Probes, 15:267- 274.
Bhandari, N., Nepali, D.B., Paudyal, S. 2013. Assessment of bacterial load in broiler chicken meat from the retail meat shops in Chitwan, Nepal. Int J Infect Microbiol. 2 (3):99-104
Capita, R., Alonso-Calleja, C., Garcia-Arias, M.T., Moreno, B. and GarciaFernandez, M.C. 2000. Effect of trisodium phosphate on mesophilic and psychrotrophic bacterial flora attached to the skin of chicken carcasses during refrigerated storage. Food Sci. Technol. Int., 6:345- 350.
Capita, R., Alonso-Calleja, C., Garcia-Arias, M. T., Moreno, B., Del Camino GarciaFernandez, M. 2002a. Methods to Detect the Occurrence of Various Indicator Bacteria on the Surface of Retail Poultry in Spain. Journal of food science. 67:2.
Chang, W.S., Afsah-Hejri, L., Rukayadi, Y., Khatib, A., Lye, Y.L., Loo, Y.Y., MohdShahril, N., Puspanadan, S., Kuan, C.H., Goh, S.G., John, Y.H.T.,Nakaguchi, Y., Nishibuchi, M., Son, R. 2013. Quantification of Escherichia coli O157:H7 in organic vegetables and chickens. International Food Research Journal 20(2):1023-1029.
Chaudhrya, M., Rashid, H., Hussain, M., Bin Rashid, H., Ahmad, A. 2011. Evaluation of bacteriological quality of whole Chcicken carcasses with and withoutskin by Comparing level of indicator bacteria . Sci. Int. (Lahore), 23(4):307- 311.
Cegielska-radziejewska, R., Tycner, B., Kijowski, J., Zabielski, J., Szablewski, T. 2008. Quality and shelf life of chilled pretreated map poultry meat products. Bull Vet InstPulawy 52: 603-609.
Chaem, H., Ahn, C., Park, B., Yon, Y., Cho, S., Choi, Y. 2002. Physicochemical properties of Korean chicken. Korean J. Poul. Sci. 29(3):185-194
Chaiba A., RhaziFilali F., Chahlaoui A., SoulaymaniBencheikh R., Zerhouni M. 2007. Microbiological Quality of Poultry Meat on the Meknès Market (Morocco). Internet Journal of Food Safety. 9: 67-71
Cohen, N., Ennaji, H., Bouchrif, B., Hassar, M., Karib, H. 2007. Comparative Study of Microbiological Quality of Raw Poultry Meat at Various Seasons and for Different Slaughtering Processes in Casablanca (Morocco). J. Appl. Poult. Res. 16:502–508
Colmegna, S., Invernizzi, A., Mascher, A. L., Corsale, E. Ferrazzi, V., Grilli,G. 2009. Microbiological characteristics of poultry meats – Results of inspections carried out in the province of Milano, Italy. Ital. J. Anim. Sci. 8:765-770.
Craven, S.E., Cox, N.A., Bailey, J.S., Cosby, D. E. 2003. Incidence and tracking of Clostridium perfringens through an integrated broiler chicken operation. Avian Dis. 47(3):707-11.
Donnelly, C.W., Brackett, R.E., Doores, S., Lee, W.H., Lovett, J. 1992. Listeria. In Vanderzant C, Spilttstoesser DF (Eds). Compendium of Methods for the Microbiological Examination of Foods 3rd Ed. Washington D.C.: American Public Health Association. Chapter 38.
FAO 2010. Poultry Meat & Eggs. Investment Centre Division.Vialedelle Terme di Caracalla, 00153 Rome, Italy.
FDA (Food and Drug Administration) 2001. Bacteriological Analytical Manual Chapter 16. Clostridium perfringens.
FDA (Food and Drug Administration) 2002. Bacteriological Analytical Manual FDA (Food and Drug Administration) 2011a. Bacteriological Analytical Manual. Chapter 5. Salmonella.
Food and Drug Administration "FDA" 2011b. Bacteriological Analytical Manual Chapter 10. Detection and enumeration of Listeria monocytogenes in Foods. Chapter 4, Enumeration of Escherichia coli and coliform bacteria.
Gonzalez-Fandos, E., Dominguez, J. L. 2006. Efficacy of lactic acid against Listeria monocytogenes attached to poultry skin during refrigerated storage.
Departamento de Agricultura y Alimentacio´ n, Area de Tecnologi´a de los Alimentos, ComplejoCienti´fico-Tecnolo´ gico, Universidad de La Rioja, Madre de Dios 51, 26006 Logron˜ o, Spain.
Gundogan, N., Citak, S., Yucel, N., Devren, A. 2005. A note on the incidence and antibiotic resistance of Staphylococcus aureus isolated from meat and chicken samples. Meat Science 69:807–810.
Hajian, S., Rahimi, E., Mommtaz, H. 2012. A 3-year study of Escherichia coli O157:H7 in cattle, camel, sheep, goat, chicken and beef minced meat. 2011 International Conference on Food Engineering and Biotechnology IPCBEE 9.
Hall, H.E., Angelotti, R. 1965. Clostridium perfringens in Meat and Meat Products. Applied Microbiology, 13:3
Harrigan, W.F. and McCance, M.E. 1976. Laboratory Methods in Food and Dairy Microbiology. Academic Press, London.
Hitchins, A.D. 2003. Detection and Enumeration of Listeria monocytogenes in Foods. Bacteriological Analytical Manual Online. U. S. department of Health and Human Services, U.S. Food and Drug Administration Center for Food Safety & Applied Nutrition. Chapter 10. On June 7, 2004 http://www.cfsan.fda.gov/~ebam/bam- 10.html.
Huong, C.T. ., Duong, N.T.H., Hien, N.T.T. 2009. Contamination of some bacteria isolated from chicken meat in retail markets in Hanoi and examination of the antibiotic resistance ability of Salmonella and E.coli strains isolated. J. Sci. Dev. 7 (2):181 – 186
ISO, 2001. International Organization for Standardization Microbiology of Food and Animal Feeding Stuffs—Horizontal method for the detection and enumeration of Enterobacteriaceae— Colony count technique. Geneva.
Javadi, A., Safarmashaei, S. 2011. Microbial Profile of Marketed Broiler Meat. Middle-East Journal of Scientific Research 9(5):652-656.
Joa, M., Kim, J., Lim, J., Kang, M., Koh, H., Park, Y., Yoon, D., Chae, J., Eo , S., Lee, J. H. 2004. Prevalence and characteristics of Escherichia coli O157 from major food animals in Korea. International Journal of Food Microbiology 95:41– 49
Joshi, N., Joshi, R.K. 2010. Bacteriological quality of meat sold in retail market in Uttar Pradesh. Journal of Veterinary Public Health 8(2):137-139.
Kalin, R., Ongor, H., Cetinkaya, B. 2012. Isolation and Molecular Characterization of Escherichia coli O157 from Broiler and Human Samples. Foodborne Pathogenes and Disease. 9:4.
Kiranmayi, C.B., Krishnaiah, N. 2010. Detection of Escherichia coli O157:H7 prevalence in foods of animal origin by cultural methods and PCR technique. Veterinary World. 3: 1.
Kitai, S., Shimizu, A., Kawano, J., Sato, E., Nakano, C., Kitagawa, H., Fujio, K., Matsumura, K., Yasuda, R., Inamoto, T. 2005. Prevalence and characterization of Staphylococcus aureus and enterotoxigenic Staphylococcus aureus in retail raw chicken meat throughout Japan. J Vet Med Sci. 67(3):269-74.
Koluman, a., Unlu, t., Dikici, a., Tezel, a., Akcelik, e., Burkan, t. Z. 2011. Presence of Staphylococcus aureus and Staphylococcal Enterotoxins in Diff erent Foods. KafkasUniv Vet FakDerg 17 (Suppl A): S55-S60
Kozacinski, L., Hadžiosmanovic, M., Zdolec, N. 2006. Microbiological quality of poultry meat on the Croatian market. Veterinarskiarhiv 76(4):305-313.
Kozacins, L., Fleck, Z. C., Kozacinski, Z. M., Ilipovic, I., Mitak, M., Bratulic, M., Mikus, T. 2012. Evaluation of shelf life of pre-packed cut poultry meat. Veterinary ski arhiv 82(1):47-58.
Lertworapreecha, M., Sutthimusik, S., Tontikapong, K. 2013. Antimicrobial resistance in salmonella entericaisolated from pork, chicken and vegetables in southern Thailand. Jundishapur JMicrobial. 6(1):36-41.
Lindblad, M., Lindmark, H., Lambertz, S.T., Lindqvist, R. 2006. Microbiological baseline study of broiler chickens at Swedish slaughterhouses. J Food Prot. 69(12):2875-82.
Mbata, T.I. 2005. Poultry meat pathogens and its Control. Department of Applied Microbiology and Brewing .Nnamdi Azikiwe University, P.M.B 5025 .Awka Nigeria. Internet Journal of Food Safety 7:20-28.
Mead, G.C. 2004. Fresh and Further-Procesed Poultry. In: Lund, B.M., BairdParker, T. C., & Gould, G.W., (Eds.). The microbiological safety and quality of fod. 3rd Ed. Gaithersburg, Aspen 453-457
Miwa, N., Nishina, T., Kubo, S., Atsumi, M., Honda, H. 1998. Amount of enterotoxigenic Clostridium perfringens in meat detected by nested PCR. Int J Food Microbiol. 21; 42 (3):195-200.
Molla, B., Yilma, R., Alemayehu, D. 2004.Listeria monocytogenes and other Listeria species in retail meat and milk products in Addis Ababa, Ethiopia. Ethiop.J.Health Dev.18(3)
Momtaz, H., Dehkordi, S.F., Rahimi, E., Asgarifar, A., Momeni, M. 2013. Virulence genes and antimicrobial resistance profiles of Staphylococcus aureus isolated from chicken meat in Isfahan province, Iran. J. Appl. Poult. Res. 22(4):913-92
Nickelson, I.R., Finne, G. 1992. Fish, Crustaceans and Precooked Seafood. In Vanderzant, C. and Spilttstoesser D. F. eds. Compendium of Methods for the Microbiological Examination of Foods. 3rd Ed. Washington D.C.: American Public Health Association.
Northcutt, J.K., Berrang, M.E., Smith, D.P., Jones, D. R. 2003. Effect of Commercial Bird Washers on Broiler Carcass Microbiological Characteristics. J. Appl. Poult. Res. 12:435–438
Nowell, V.J., Poppe, C., Parreira, V. R., Jiang, Y., Reid-Smith, R., Prescott, J. F. 2010. Clostridium perfringens in retailchicken. Anaerobe 16:314–315
Omorodion, N. J. P. N., Odu, N. N. 2014. Microbiological Quality of Meats Sold In Port Harcourt Metropolis, Nigeria. Nature and Science. 12(2).
Paiao, F.G., Arisitide , L.G. A., Murate, L.S., Vilas-Boas, G.T., Vilas- Boas, L.A., Shimokoma , M. 2013. Detection of Salmonella spp, Salmonella enteritidis and Typhimurum in naturally infected broiler chickens by a multiplex PCRbased assay. Brazilian J. Microbiol. 44:37-14.
Rindhe, S.N., Zanjad, P.N., Doifode, V.K., Siddique, A., Mendhe, M.S. 2008. Assessment of microbial contamination of chicken products sold in Parbhani city. Veterinary World, 1(7): 208-210.
Ruban, S.W., Fairoze, N. 2011. Effect of Proceesing Conditions on Microbiological Quality of Market Poultry Meats in Bangalore, India. J. of Animal and Veterinary Advances. 10 (2): 188-191
Santosh Kumar, H.T., Pal, U.K., Kesava Rao, V., Das, C.D., Mandal, P. K. 2012. Effects of processing practices on the physico-chemical, microbiological and sensory quality of fresh chicken meat. Int. J. Meat Sci., 2:1-6.
Selvan, P., NarendraBabu, R., Sureshkumar, S., Venkataramanujam,V. 2007. Microbial quality of retail meat products available in Chennai city. American Journal of Food Technology 2(1):55-59.
Sengupta, R., Das, R., Ganguly, S., Mukhopadhayay, S. K. 2012. Commonly occurring bacterial pathogens affecting the quality of Chicken meat. International J. of Chemical and Biochemical Sciences 1:21-23
Shaltout, F. A. 2009. Microbiological Quality of Chicken Carcasses at Modern Poultry Plant. Third Inter. Sci. Conf., 29 Jan.- 1 Feb./ 2009, Benha & RasSudr, Egypt Fac. Vet. Med. (Moshtohor), Benha Univ.
Singh, R.V., Bhilegaonkar, K.N., Agarwal, R. K. 2005. Studies on occurrence and characterization of Clostridium perfringens from selected meats. J. of Food Safety 25:146–156.
Thangamani, A., Subramanian, S. 2012. Prevalence of Clostridium perfringens in the Chicken Meat Rendered at Retail Outlets of Namakkal, Tamilnadu. J. of Advanced Veterinary Research. 2:157- 159
USDA, 2011. Quantitative Analysis of Bacteria in Foods as Sanitary Indicators January 2011.
Vaidya, V. M., Paturkar, A. M., Waskar, V. S., Zende, R. J., Rawool, D. B. 2005. Detection of indicator organisms on poultry carcass sites in an organized slaughterhouse. J. Muscle Foods, 16:289–297.
Yeh, E.T. 2004. Master of Science. Characterization of listeria monocytogenes isolated from organic retail chicken. Faculty of the Graduate School of the University of Maryland.
Zhang, L., Davis, M.A., Conner, D.E. 2001. Poultry-borne pathogens: plant considerations. Poultry meat processing Chap. 9. CRC Press LLC, New York, USA.