I. INTRODUCTION
Using AI, the services of a single superior male can be extended to a large number (more than 250) of females instead of 8 hens (natural mating). AI in avian species expresses better fertility than natural mating (Saeki and Nagomi, 1964, Mohan et al., 2016). AI increases overall fertility and hatchability with reduced cost of production per day old chick (Brillard, 2003). Adopting AI needs fewer males which saves feed, labour, space, maintenance and operating costs but requires the immediate dilution of chicken semen because it is highly concentrated, containing 6 (roosters) to 12 (toms) billion spermatozoa/ml (Donoghue and Wishart, 2000) and has a low volume. There is the risk of sperm being killed by dehydration resulting from evaporation of water at room temperature from highly concentrated semen unless semen is diluted (Lake and Stewart, 1978) and semen diluent can increase the number of hens that can be inseminated by the semen provided by a single superior male.
Various semen diluents are available as reported in the literature but CARI poultry semen diluent produces good results (Beulah, 2017). Therefore, CARI diluent developed in our laboratory (Mohan et al., 2017) was selected to investigate the appropriate dilution rate of this diluent for optimum fertility. Further, the fertilizing ability of this diluent was also examined at 0, 24 and 48 hrs of storage of diluted semen.
II. MATERIALS AND METHODS
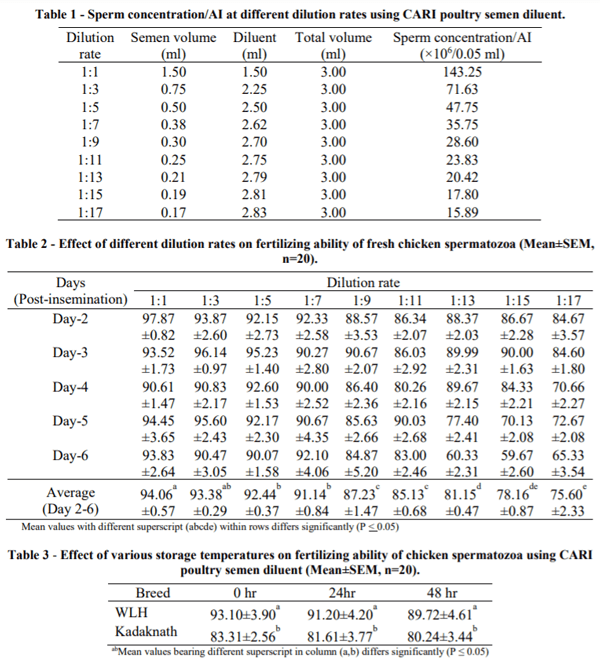
Healthy adult males (20) and females (180) from the same hatch of WLH chicken were taken randomly and maintained in individual cages under uniform husbandry conditions. Good quality semen samples were collected by the Burrows and Quinn (1937) method. For examining the fertility in fresh samples, pooled semen (sperm concentration 5.73×109 /ml) was placed in 9 round bottom glass tubes of 5 ml capacity (length=7cm, diameter =1cm). All the tubes containing varying semen volume were diluted with CARI diluent at the rate of 1:1, 1:3, 1:5, 1:7, 1:9, 1:11, 1:13, 1:15 and 1:17 to achieve the final fixed volume of 3 ml in each tube (Table 1). Immediately after dilution, the sperm concentration was examined using a haemocytometer.
Artificial insemination with freshly ejaculated semen was carried out by intravaginal insemination using an AI gun (IMV, France) in 9 different groups each of 20 hens. Fertile eggs were collected from 2-6 days of post insemination of hens and examined by candling at the 9th day of incubation. Break out studies were performed for the fertility assessment where fertility was not clear by candling. In another study, 20 healthy adult males and 60 females from the same hatch of WLH (exotic) and Kadaknath (native) chicken were taken randomly and maintained in individual cages under uniform husbandry conditions as in the above experiment. All hens in this study were divided equally into 3 groups (20 each). The first group of hens received the freshly ejaculated semen (0 hr stored) diluted (1:2) with CARI poultry semen diluent and served as control. The second and third groups of birds received the semen diluted (1:2) with CARI poultry semen diluent and stored in 5 ml capacity glass vial at 8±1°C for 24 and 48 hrs respectively before insemination of hens. A.I. and evaluation of fertility were conducted as described in experiment 1. Data were analysed as per the standard methods.
III. RESULTS AND DISCUSSION
Data on the effect of different dilution rates on fertilizing ability of freshly ejaculated chicken spermatozoa are presented in Table 2. Superior fertility was obtained from 2 to 6 days post insemination for the dilution rate of 1:1 (143.00), 1:3 (71.50) 1:5 (47.75) and 1:7 (35.75 million (m) sperm/AI) which ranged from 91 to 94%. With higher dilution rates i.e. 1:9 (28.60 m sperm/AI) and 1:11 (23.83 m sperm/A.I.), fertilizing ability of sperm reduced to 87.23 and 85.13% respectively. Further increases in the dilution rate like 1:13 (20.42 m sperm/AI), 1:15 (17.88 m sperm/AI) and 1:17 (15.89×106 m sperm/AI), reduced fertility further at 81.15, 78.16 and 75.60% respectively. According to this study, a minimum 36 million sperm (1:7) are required for good fertility in chickens. The results of this study are similar to the work carried out by Beulah (2017), who used freshly ejaculated diluted chicken semen with CARI diluent and recorded above 90 % fertility up to 1:8 (29.70×106 sperms/A.I.) dilution rate and 79% fertility at 1:18 (14.70 m sperm/AI). The reduction of fertility with higher dilution rate is associated with the decrease in number of spermatozoa per unit volume. The minimum number of sperm required per AI is 45 to 90 million /hen for good fertility in chickens (Rowell and Cooper, 1960, Kim et al., 1974) although Etches (1996) advocated 100 million sperm /hen/AI. Results on the effect of CARI diluent on storage of chicken semen at 24 or 48 hrs are presented in Table 3. In the exotic breed of chicken, fertility rates at 0 (fresh/ control), 24 and 48 hrs were 93.10±3.90, 91.20±4.20 and 89.72±4.61% respectively and in native breeds 82.31±2.56, 81.61±3.77 and 80.24±3.44% respectively. These data indicate that no significant difference was found in fertility among the three storage periods in both breeds. However, a lower (P < 0.05) fertility was found in Kadaknath as compared with the WLH breed. This may be due to breed to breed variation. Mohan et al. (2011) also reported higher fertility in WLH than Kadaknath. Poor fertility in native fowl during storage of spermatozoa may be associated with more dead and morphologically abnormal spermatozoa than for WLH. High fertility depended more on semen quality than semen quantity (Kamar, 1960). This investigation indicated that chicken semen can be stored (8±1°C) for 24 or 48 hrs in CARI diluent and still express high fertility similar to the freshly ejaculated semen (Table 3). However, earlier studies indicated that chicken semen can be stored for 24 hrs at 0-5°C (Lake, 1960, Van Wambeke, 1967, Sexton, 1977, Lake and Ravie, 1979). This suggested that the composition of the CARI poultry semen diluent may differ from others and preserve the chicken semen at 8±1°C (instead of 0-5°C) up to 48 hrs without impairing the fertility (89.72±4.60%). Adequate data are not available on 48 hr stored semen to compare with ours. However, Lake (1960) reported 47 % fertility after 48 hrs storage of semen at 0-2°C, nearly half that of the present study.
IV. CONCLUSION
Using CARI diluent, freshly ejaculated chicken semen can be extended up to 1:7 without significant loss in fertility. This diluent can be used for 24 or 48 hrs storage of chicken semen with high fertility.
ACKNOWLEDGMENTS: The authors are thankful to the Indian Council of Agricultural Research (ICAR) and Director, ICAR-CARI for providing necessary facilities to carry out this research work.
Abstract presented at the 30th Annual Australian Poultry Science Symposium 2019. For information on the latest edition and future events, check out https://www.apss2021.com.au/. 









