Introduction
Streptococcus suis (S. suis) is a major porcine pathogen responsible for important economic losses to the swine industry. In fact, it is one of the main causes of bacterial death in post-weaned piglets, from 5 to 10 weeks of age. According to the Canadian Swine Health Information Network, S. suis-related diseases are the most common infectious problem reported in Canadian swine farms. In addition, the Monitoring and Analysis Working Group from the Swine Health Information Center (SHIC) reviewed and established final rankings for what is now the Swine Bacteria Disease Matrix. As stated on its website, S. suis leads the list as the most important bacterial swine pathogen (https://www.swinehealth.org/swine-bacterial-disease-matrix/). Clinical features of these infections in pigs are meningitis, arthritis, endocarditis, polyserositis and septicemia with sudden death. The implication of S. suis as a primary respiratory pathogen has been seriously questioned, and it is now considered as a secondary agent of pneumonia. Outbreaks of S. suis disease result in decreased performance and increased mortality, which have a significant economic impact.
It is also a zoonotic agent and the infection in humans has attracted a high level of attention in last years, with deadly outbreaks reported in Asian countries, such as China and Thailand. There are at least 35 serotypes, based on capsular polysaccharide (CPS) antigens or CPS-related genes, although some of them have been proposed as being part of different bacterial species. Indeed, serotypes 20, 22 and 26 have been re-classified as Streptococcusparasuis, serotypes 32 and 34 as Streptococcusorisratti and serotype 33 as Streptococcusruminantium. Previously described S. suis serotypes 20, 22, 26, 32 and 34 are still recovered from diseased pigs and many laboratories do still identify such isolates as S. suis. Serotype 33 reference strain was originally isolated from an ill lamb (arthritis). So far, there is no single strain (confirmed by PCR) of this serotype recovered from swine: the few field strain reported in the past were identified by coagglutination (serological assay), a technique that presents many cross-reaction. When tested by PCR, these field strains were confirmed as being untypable or autoagglutinating. On the other hand, Streptococcus ruminantium (some of them detected as S. suis serotype 33 and others not) are frequently recovered from diseased ruminants (bovine and ovine) suffering from respiratory disease, abscess, arthritis, mastitis and other types of infections. S. ruminantium (and previous S. suis serotype 33) should not be considered as a primary porcine pathogen anymore.
Among the serotypes described, type 2 is the most virulent and frequently isolated from both diseased pigs and humans. Serotype 9 is another important serotype involved in swine diseases However, this is the reality of Europe, which may be different in America, especially Canada and USA. Phenotypically and genotypically different strains of S. suis serotype 2 (with different degree of virulence) have been isolated in different parts of the world. S. suis strains have also been analyzed and classified into clonal complexes (CC) composed by different sequence types (ST) when analyzed by multilocus sequence typing and, more recently, by whole genome sequencing, confirming the genetic heterogeneity within the S. suis species. Finally, untypable strains are also sometimes isolated from diseased animals and their possible virulence capacity should not be disregarded.
Pigs may acquire S. suis from the sows (during farrowing and also through oral/nasal contact) and through piglet-to-piglet transmission. Bacteria are localized in the tonsils, but they are also present in saliva: most pigs are carrier animals, harboring mostly low virulent strains. In the presence of virulent strains, some carrier piglets will eventually develop septicemia, meningitis and/or arthritis due to dissemination of S.suis, when maternal antibodies decline (between 5 and 9 weeks of age). The incidence of the disease is usually kept under 5% in the field. However, in the absence of prophylactic, metaphylactic and/or curative antibiotic treatments, mortality may reach 20%. Indeed, a significant increase of S. suis-related disease in post-weaned piglets has been observed in the last years, mainly associated to the reduction in the use of antibiotics. Although studies on S. suis have been significantly increased in the last 15 years, there are still many unresolved questions.
S. suis invades through the respiratory tract, gastrointestinal tract or both?
An overview of the pathogenesis of the infection can be observed in Figure 1.
It has always been accepted (and demonstrated) that S. suis enters through the respiratory tract. Bacteria are then located at tonsils and remain there, being S. suis a normal inhabitant of the upper respiratory tract. Under stressful conditions and in the absence of antibodies (post-weaned piglets), potentially virulent strains invade the bloodstream (by still unconfirmed mechanisms) and induce bacteremia, septicemia and clinical signs (depending the colonized organs), through the induction of high levels of pro-inflammatory mediators. Sometimes, concentration of such mediators is so high that sudden death occurs and animals are simply found dead.
Figure 1: Proposed pathogenesis of the infection caused by Streptococcus suis
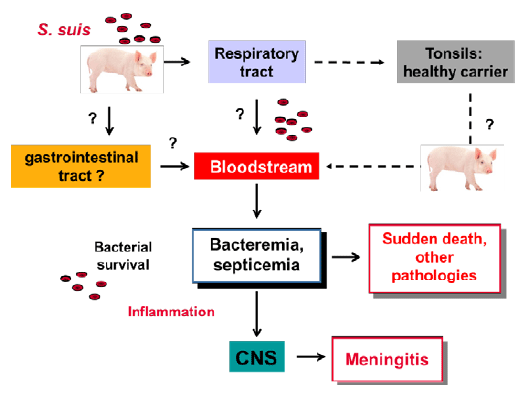
More recently, a new route of entry has been proposed: the oral route, as it happens very often in humans (South-East Asia). However, this still needs to be confirmed. Studies reproducing disease mimicking this route of infection (for example: intestinal translocation) used either direct inoculation of high concentration of virulent S. suis in the jejunum or high concentration of bacteria included inside acid-resistant capsules and then given orally to pigs. Even under these circumstances, a very low number of infected animals developed disease. When post-weaned animals were challenged through the oral route (without acid-resistant capsules), animals remained healthy. So far, many studies showing presence of S. suis in the intestine used DNA detection, so it is not easy to differentiate between live and dead bacteria. In addition, many of these studies included the use of genes that are not able to differentiate between S. suis and S. suis-like microorganisms. However, other studies isolated S. suis from feces. Indeed, in some cases of septicemia, animals may present diarrhea and S. suis (the strain responsible for the disease) can be isolated from feces. However, it is not clear if the simply presence of virulent strains in the intestine of clinically healthy pigs is enough to induce disease. It is important to note that stress due to weaning and feed changes may significantly modify the intestinal environment causing stress to animals, which may be much susceptible to develop disease. Usually, first animals to develop disease are those in great shape and the biggest ones. Poor adaptation to solid feed may be even more important in such animals. Stress may induce invasion of S. suis into the bloodstream, but this may also happen from tonsils. More studies about possible pathogenesis of the infection through the gastrointestinal tract are needed before proposing new food additives to control S. suis infections (see below).
What is the definition of virulent strains of S. suis?
The majority of porcine S. suis infections are caused by strains belonging to a relatively small number of serotypes. Although the distribution of serotypes from clinical cases differs depending on the geographic location, serotype 2 strains are responsible for the majority of cases in both swine and humans worldwide, and thus this serotype has been historically considered the most frequent and virulent type. However, this is true for Europe and Asia. In addition, serotypes 1 and 14 have also been described as highly virulent. Indeed, it has been shown that highly virulent strains of serotypes 1, 2 and 14 in Western countries are mostly included in CC1 (mostly ST1). These strains possess some virulence markers such as the suilysin (SLY), the muramidase-released protein (MRP) and the extracellular protein factor (EF), and presence of genes coding for such proteins can be detected by PCR and this is used in routine diagnosis in some laboratories. Besides this serotype, recent years have seen the emergence of serotype 9 strains among swine diseases in several European countries. Most of these strains isolated from diseased pigs (and differently from those recovered from tonsils of healthy pigs) belong to specific STs (such as ST16, ST123 and ST125) and produce SLY and a variant of the protein MRP. Interestingly, it is not easy to reproduce disease with such “virulent” serotype 9 strains and usually highly aggressive intravenous or intratracheal infections are needed. Intranasal infection does not usually induce disease, even with high susceptible caesarian-derived colostrum deprived piglets.
What happens with strains isolated in America? One striking observation is that the percentage of S. suis serotype 2 strains recovered from diseased pigs is lower in North America than in other parts of the world. In addition, human S. suis disease cases are rarely reported in Canada and USA. In fact, it has been shown that serotype 2 strains in North America are less virulent and genetically unrelated to those causing disease in other parts of the world, such as Europe and Asia. Serotype 9 strains are also different and belong to a bunch of different STs, not usually associated with disease. Highly virulent serotypes 1 and 14 (CC1, ST1) are, however, present in these two countries. On the other hand, most isolates from diseased pigs belong to different serotypes (such as serotype 1/2, the most prevalent in USA). Analysis of virulence markers (MRP, EF and SLY) does not give any additional information since most serotype 2 strains are negative for SLY and EF and serotype 9 strains are negative for SLY. In Latin America, there is not much information and the presence of highly or lower virulent strains of serotype 2 may depend on the source (geographical region) of genetics. Data from Argentina (a country with more of 20 human cases described, the highest number of cases in whole America) indicate that highly virulent serotype 2 CC1 (ST1) strains are widely present, similar to what is observed in Europe. Data from Brazil, the most important swine producer in Latin America, are missing. In addition, no human cases have been reported, which may indicate a problem of misidentification in laboratories of human medicine.
Do S. suis-related diseases depend on the presence of virulent strains only?
S. suis-associated diseases are complex. The presence of a potential virulent strain alone does not guarantee appearance of clinical signs and, sometimes, clinical signs are observed in the absence of such strains. Virulence of strains belonging to serotypes other than serotypes 1, 2, 9 and 14 is almost unknown, there are no virulence markers and there is no validated model of infection in pigs, since in most cases, animals infected with strains belonging to other serotypes will not develop disease. However, these strains are commonly found in S. suis-associated diseases, North America, respectively. This is still one of the major challenges we face in the diagnosis of S. suis infections: how to determine if a given strain (from non-serotypes 1, 2, 9 or 14) isolated from a diseased animal is, in fact, really responsible for most clinical cases in a farm. We usually recommend performing a necropsy of at least 3 animals from the same batch, in 3 different batches. If S. suis is isolated in pure or predominant culture from internal organs (other than lungs) in most of these animals, and serotyping indicates that one or a very few serotypes are always involved, we can conclude that those strains are probably important. If inconclusive results are obtained, additional animals should be analyzed: if at the end, 4-5 or more serotypes are detected, predisposing factors should be taken into consideration before planning the use of an autogenous vaccine (see below). The analysis of the results is even more complicated if untypable strains are involved: are these all the same strain? Or different untypable strains are causing disease? These questions can only be answered through the sequencing and comparison of such strains.
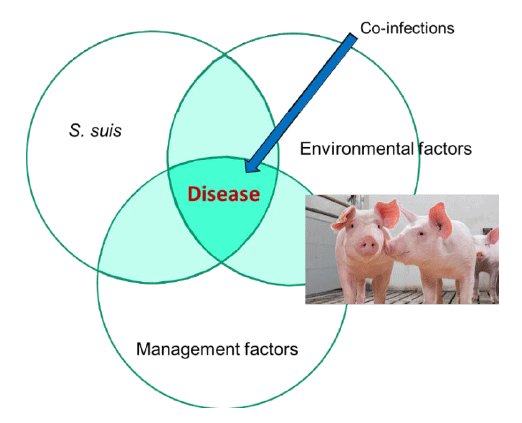
In fact, when multiple strains are involved, other factors may influence the presence of disease (Figure 2). However, S. suis is also the disease of the exceptions, since everything may happen. In rare conditions, 3-4 different virulent strains affecting animals from the same herd (example: serotypes 1, 2 and 14, all ST1, or a serotype 2 ST1 and a serotype 9 ST16) may also occur and this complicate even more the diagnosis. We have recently observed animals dying at the same time with pure culture isolation of a serotype 2 (one animal) and a serotype 14 (the second animal), both virulent strains.
Obviously, the presence of virulent strains is an important factor. However, concomitant infections with other pathogens may highly contribute to the presence of clinical signs. The most important, by far, is the instability of the farm to Porcine Reproductive and Respiratory Syndrome (PRRS) virus. Although data from the field clearly show that a PRRSv previous infection predisposes to clinical disease caused by S. suis, the exact mechanisms involved are still unknown. It has been also shown that previous infection with Aujeszky’s disease virus may predispose animals to an enhanced S. suis disease. Swine influenza virus (SIV) may also enhances the infections caused by certain serotypes of S. suis which possess sialic acid in their capsule: serotype 2 but also serotypes 1, 1/2 and 14. How this infection may influence disease caused by other serotypes (which do not have any sialic acid in their bacterial surface) is unknown. Unfortunately, there are no scientific data concerning other co-infections, such as those caused by porcine circovirus, mycoplasma or others. Co-infections (with clinical disease) with Haemophilus parasuis seem to be rare. The presence of mycotoxins has also been suggested as a predisposing factor but it has never been scientifically studied.
It is also considered that environmental factors may greatly influence the appearance of S. suis-related diseases, such as (among others) poor ventilation, high humidity, inadequate sanitation and important temperature variation between night and day. It is interesting to comment here how experimental infections by the intranasal route are done with virulent strains. If a high concentration of a virulent strain of S. suis serotype 2 is inoculated to the nasal cavities of conventional pigs, usually few or no clinical signs are observed. A previous treatment done with acetic acid to irritate the nasal mucosa should be done, followed by the infection with the virulent strain. Under these conditions, clinical signs may be observed, and this will depend on the farm from where the animals originated. This indicates that other environmental factors, such as high levels of dust and ammonia (causing irritation), may greatly influence animal susceptibility to develop clinical signs. Chances to reproduce disease will considerably increase if some kind of stress is applied to the infected animals. Although S. suis may be clearly a primary etiological agent of disease, the Koch’s postulates sometimes are not easy to be reproduced with this difficult pathogen.
Figure 2: Factors contributing to the expression of S. suis-related diseases
Management factors may also influence the development of S. suis-related diseases. For example, high level of cross fostering, overcrowding, teeth clipping and tail docking (arthritis), ear notching (arthritis), mixing pigs of different ages, poor adaptation to solid feed in the nursery and low levels of vitamin E. It has been also suggested that the use of strong antibiotics given at the first week of life may been associated with the increased presence of clinical signs due to S. suis.
Finally, our studies have demonstrated that the immunological status of animals is extremely important. Indeed, most clinical cases occur when maternal antibodies decline (between 5 and 9 weeks of age) and before natural antibodies are produced. Indeed, natural antibodies slowly increase from 9-10 weeks of age: nobody knows if such antibodies are directed specifically against S. suis or to other antigenically-related bacteria present in tonsils. These antibodies are always present in pigs (even in the absence of S. suis-related clinical disease in post-weaned animals), increase with age and are probably protective. This may explain why clinical cases are rarely observed in adult animals.
Does S. suis represent a danger for antibiotic resistance?
As mentioned before, the incidence of the disease is usually kept under 5% in the field, but this is mainly due to the extensive and routine (where allowed) prophylactic and metaphylactic use of antibiotics. In some countries, where antibiotics in feed are banished, these are sometimes used in water. The problem of the antibiotic use for S. suis infections is not necessarily the development of resistance of such strains to the most frequently molecules used to treat S. suis-affected pigs. The antibiotics of choice for this type of infection are beta-lactams. Most strains able to cause disease are susceptible to such drugs and this is due to the mechanism of resistance: it is not mediated by enzymatic degradation of the beta-lactam molecules (beta-lactamases), but rather involves modifications in the form of altered molecular weight and/or a decrease in the penicillin-binding capacity of its beta-lactam target proteins: the penicillin-binding proteins (PBP). Indeed, an increase in resistance to penicillin and amoxicillin by S. suis is brought by distinct cumulative alterations in its PBPs which happen at the chromosomal level. The consequence of such mechanism of resistance is that it may take years to develop some kind of resistance and, if it develops, it progresses very slowly. On the other hand, it is important to keep testing field strains to confirm such an hypothesis.
It is important to note that some diagnostic laboratories do not use standardized methods to measure resistance, so equivocal results are sometimes observed, as for example: sensitivity to penicillin but resistance to amoxicillin or resistance to amoxicillin but susceptibility to amoxicillin with clavulanic acid: this is simply unlikely to be true. If some kind of resistance to beta-lactams is observed, it is recommended to send the isolate to an independent laboratory to repeat the test. Some strains isolated from tonsils may present lower level of susceptibility, event to beta-lactam antibiotics: most of these strains are non-encapsulated and non-virulent. In addition, many of these bacteria do not even belong to the Streptococcus suis species.
So, can we say that antibiotic resistance is not important for S. suis? It should be remembered that some of the antibiotics used are also of importance for human medicine. In addition, worldwide data from resistance of S. suis to antibiotics are alarming. Field strains are highly resistant to many different antimicrobials (such as tetracyclines and erythromycin), even if they are still susceptible to beta-lactams. Indeed, S. suis is considered a niche for antibiotic resistance and represents a high risk of transmission of resistance to other pathogens. This arises from mobile genetic elements in S. suis carrying resistance genes that are transferable at high frequency not only between S. suis strains but also to other bacterial species. Again, it is important to emphasize the need for continuous surveillance of resistance patterns in all pig pathogenic bacteria.
How to prevent disease caused by S. suis without using antibiotics?
Restrictions in the use of antibiotics brought, among other consequences, an increase of clinical disease in post-weaned piglets. In farms where animals are raised without antibiotics, S. suis is one of the most important concern. The question everybody is asking: how can we prevent S. suis diseases? Everybody also agrees that controlling stress and predisposing factors (concomitant infections, environmental and management factors, etc.) may significantly help to reduce disease. However, this is frequently not enough. What else can be done?
There are many alternatives to antibiotics that have been tested for S. suis. However, most of them have been tested in vitro but not in vivo (Table 1). So far, no active compound has been clearly demonstrated as being effective to control S. suis disease in vivo.
Table 1: Different products tested as an alternative to antibiotics to kill S. suis
The use of feed additives became also popular, based on the hypothesis of S. suis causing disease through the intestinal route, as discussed above. However, most available studies either have been tested in vitro or have not been published in peer-reviewed journals and lack the strict evaluation from the scientific community. Some examples are present in Table 2. Again, the use of these products become more and more popular, but there is no scientific proved data indicating any advantage to use them to control and prevent S. suis infections. More controlled and scientific research is indeed necessary.
Table 2: Feed additives tested to control S. suis infections

As the complexity of S. suis epidemiology in swine increases (multiple strains, multiple serotypes), field reports describing difficulty in disease control and management are common. A logical alternative is the use of vaccines. However, so far, there is no commercial vaccine able to protect against all serotypes/strains of S. suis. Many research studies evaluated sub-unit vaccines (proteins) or even live vaccines, but controversial results have been obtained. The consequence is that the only alternative practitioners have in hands is the use of bacterins (killed whole bacteria), mostly autogenous vaccines. Autogenous vaccines are bacterins based on the predominant strain(s) recovered from diseased pigs in the affected farm and produced by accredited laboratories. Most published studies have been done with bacterins produced in research laboratories with reference strains, a kind of artificial “autogenous vaccine”, not produced by accredited laboratories.
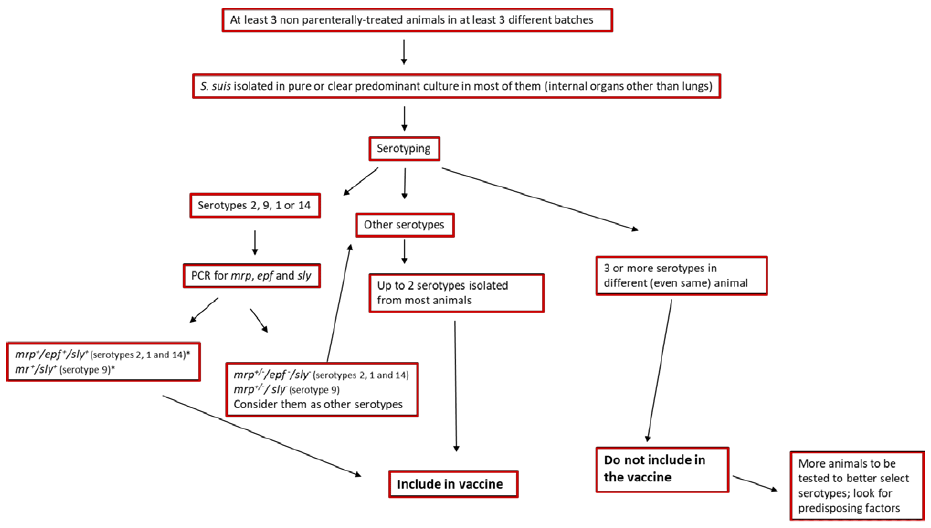
For the production of an autogenous vaccine, the first step is the choice of the strain. Differently from Glaesserella (Haemophilus) parasuis, S. suis is easy to isolate. However, under certain circumstances, different strains may be isolated from diseased pigs within the same farm (see above4). S. suis may be either a secondary or primary pathogen: as mentioned, co-infections, environmental or management issues may help moderately virulent S. suis strains, normally located in tonsils, to induce disease. In these cases, it is better to concentrate the efforts to reduce predisposing factors, as S. suis disease is a consequence not really a cause of the health problem. Diagnosis of S. suis infection as primary pathogen is not easy. Figure 3 shows a standard procedure that may help on the decision to incorporate a given strain to an autogenous vaccine.
Figure 3: Proposed methodology to choose strains to be included in an autogenous vaccine. *For European strains only.
In some farms, autogenous vaccines include 4-5 serotypes of S. suis and sometimes other bacterial species, such as Staphylococcus hyicus, Streptococcus dysgalactiae, Glaesserella (Haemophilus) parasuis, Erysipelothrix rhusiopathiae and Actinobacillus suis. Although never studied, the inclusion of such huge mass of antigens may have two different consequences: a) the reduction of the bacterial concentration of each individual strain to keep a 2 ml vaccine, and/or b) distraction of the immune system. It is hard to evaluate if all strains are necessary and if the immune system is able to produce antibodies to all relevant antigens. Finally, the production of autogenous vaccines is more an “art” than a “science”. The value of autogenous vaccines, at large, cannot be evaluated. Why? Because each company produces the vaccine differently, and most of the variables have never been studied. Some of the variations that may happen among different vaccine productions are presented in Table 3.
Table 3: Some variables that may be present when producing an autogenous vaccine.
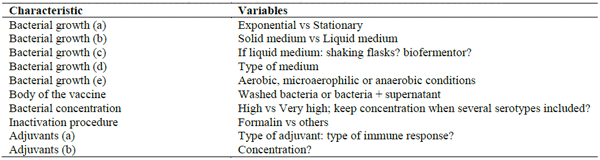
Indeed, under each condition of vaccine production, different antigens may be expressed. In addition, it has also been demonstrated that adjuvants used may greatly influence the protection observed with experimental vaccines. So, it is impossible to compare autogenous vaccines produced by different companies. This explains in part why there are almost no scientific data evaluating autogenous vaccines in general in the field, at least published by peer-reviewed journals. Most data are from internet or oral presentations in different congresses and other meetings. In addition, most studies do not include control groups: the contribution of the autogenous vaccine to the control of the infection is normally evaluated by studies done “before” vs “after”, where mortality and the use of antibiotics to treat animals are compared. One of the problems is that, normally, “mortality” refers to total mortality in the nursery…not necessarily mortality related to S. suis only. In addition, other measures to control predisposing factors may also be applied simultaneously with vaccination, which may complicate the analysis. On the other hand, the inclusion of a control group may not solve the problem: when mortality is under 5%, a significant high number of animals must be included in both groups, since otherwise it is difficult to observe differences. Finally, there are no studies where the antibody response of vaccinated animals with a commercial autogenous vaccine have been evaluated, so it is unknown if such vaccines are able, at least, to induce an increase of the antibody levels, which would be an indirect way to study potential protection.
As mentioned, adjuvants can dramatically influence the vaccine-induced antibody response, as it was studied with experimental (not commercial) vaccines. Not all antibodies (immunoglobulins or IgG) induced by a bacterin are indeed protective. Some IgG subclasses (called “isotypes”), such IgG2 and to a lesser extent IgG3, are particularly effective at mediating bacterial ingestion and destruction by leukocytes (phagocytosis). Indeed, S. suis resistance to phagocytosis and thus innate immunity clearance, is lost in the presence of antibodies that promote bacterial phagocytosis and destruction by professional phagocytes (called “opsonophagocytosis” or OPA test). Other antibodies isotypes, such as IgG1, even if they are induced after vaccination and recognize the pathogen, are much less protective, since they cannot help the host to destroy the pathogen. Interestingly, different adjuvants may influence the production of “protective” or “non-protective” IgGs. An in vitro OPA test has been lately standardized to measure protective activity of antibodies against S. suis, although it has never been used so far to measure the antibody response against field autogenous vaccines. So, not only levels of antibodies should be measured, but also their functionality. We are presently developing such tests in our laboratory and results will be available soon.
Finally, there are no clear data when and how autogenous vaccines should be applied, and there are almost no scientific studies available. In the field, and without any scientific data, autogenous vaccines are used either in sows or piglets or (less common) both. Vaccination of sows before farrowing might elicit passive maternal immunity, being less costly, and thus representing an economical alternative to piglet vaccination. Yet, available results indicate that prefarrowing immunization of sows with these experimental bacterins to protect piglets gave inconclusive results with either no antibody production, antibody production that did not protect piglets or a restricted protection for piglets of less than 6 weeks of age. In fact, piglets at nursery remain without antibodies, a period of high risk of S. suis disease in many farms. Active vaccination of young animals, such as suckling piglets, has the concern of possible interference with maternal antibodies. Indeed, neither vaccination of suckling nor of weaning piglets from immunized sows with experimental bacterins was constantly associated with a prominent active immune response and protection at 8 weeks of age. In this regard, interference between maternal antibodies and active production of antibodies against S. suis was also suggested in a field study with also an experimental bacterin. Vaccination of older piglets (for example, at 3 and 5 weeks of age) may not induce a booster antibody production early enough to protect piglets at the nursery: there is clearly a problem of a window of vaccination. Knowledge of antibody kinetics is thus required before implementation of a rational vaccination program. The adopted strategy should allow minimal interference between passive maternal immunity and active immunization in piglets but maximal protection for pigs at the approximate time of onset of clinical signs.
Conclusions
S. suis infections are multifactorial and very difficult to control. With the reduction in the use of antibiotics, nursery mortality due to this pathogen significantly increased. S. suis may be a primary or secondary pathogen and control of predisposing factors should not be neglected. Evaluation of the virulence of the involved strains is not easy to perform under all circumstances; in addition, the virulence potential of the strain is only one of the aspects to be taken into consideration to control disease. Products that can be used as alternatives to antibiotics still need to be scientifically evaluated. Finally, due to limited field reports concerning immunogenicity and protection, the usefulness of autogenous bacterins in a rational vaccination program remains to be proved and needs further research. Studies, where independent researchers evaluate the influence of different aspects of the production of an autogenous vaccine on their protective capacity, are also required. As it is still the only option available to be used as immunogens, a diagnostic effort on the evaluation of the strains to be included in such vaccine must be done. Finally, autogenous vaccines must be produced by companies having a large experience on this field.
Published in the proceedings of the International Pig Veterinary Society Congress – IPVS2020. For information on the event, past and future editions, check out https://ipvs2022.com/en. 












.jpg&w=3840&q=75)