Mycotoxins in Feed Grains and Ingredients
Published: June 3, 2008
By: Tim Herrman and Dionisia Trigo-Stockli, Extension State Leader, Grain Science and Industry - Kansas State University
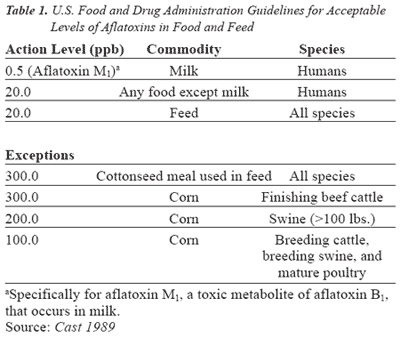
Mycotoxins also pose a potential hazard to human health. The Food and Drug Administration (FDA) has established an action level of 0.5 parts per billion (ppb) of the mycotoxin, aflatoxin M1 in milk for humans and 20 ppb for other aflatoxins in foods other than milk. Dust emitted while handling contaminated grain and ingredients also may contain mycotoxins, thereby posing a health threat to individuals who inhale these contaminants.
Aflatoxins are the most notorious mycotoxin due to their early discovery and the abundant amount of research information available compared to other mycotoxins. These toxins were isolated and identified in 1961, following a 1960 incident in which 100,000 turkey poults in the British Isles died from eating feed containing contaminated peanut meal. Aflatoxin may occur in the field and in storage.
Droughts and accompanying high temperatures during grain fill are conditions conducive to fungal invasion and mycotoxin production in corn. Notable epidemics in corn during the past decade include 1993 in the southeastern U.S. and in 1988 in the corn belt, both accompanied by droughts and high summer temperatures.
Producers and feed manufacturers may be less familiar with other mycotoxins such as ochratoxin, zearalenone, deoxynivalenol, T-2, and fumonisin. Each of these mycotoxins are produced by one or several species of fungi when conditions are favorable for growth and toxin production.
Mycotoxins can result in significant economic loss for crop producers and grain handlers who receive market discounts for contaminated grain or, in a worst case scenario, have to dispose of severely contaminated grain. Feed manufacturers who incorporate mycotoxin-contaminated grain or feed ingredients in their product may
encounter livestock health problems or poor animal performance.
Potential economic losses include a loss of business, product recall, and product liability. Farmers who feed their own contaminated grain to livestock can incur financial loss, including poor animal reproduction, growth suppression, and animal death. The following bulletin discusses the cause, clinical signs of mycotoxicoses, government regulations, reconditioning/detoxification strategies, and detection technology associated with mycotoxins in grain.
Aflatoxin
Aflatoxins are produced by the fungi Aspergillus flavus and A. parasiticus. These fungi are present in soil and decaying plant material, cause heating and the decay of stored grain, and may invade corn in the field. Crops and feed ingredients most susceptible to fungi and aflatoxin development include corn, peanuts, peanut meal, cottonseed, and cottonseed meal.
Conditions that favor the invasion of corn by Aspergillus flavus in the field include drought stress or damage to the corn ear by ear worms or other insects, birds, hail, or early frost. High temperatures, high relative humidity around the kernels, and kernel moisture below 30 percent (wet basis) are ideal conditions for fungal invasion of the kernel.
The optimum temperature for aflatoxin production in storage is between 25ºC and 32ºC (77ºF and 90ºF). Kernels with a moisture content below 15 percent are at less risk of mold growth and aflatoxin production, while an optimum kernel moisture is around 18 percent and an optimum relative humidity in the bin is 85 percent or higher.
Clinical Signs of Aflatoxicosis: The clinical signs of aflatoxicosis are extremely varied. Aflatoxicosis in swine is primarily a liver disease although other organ systems may be involved. Decreased growth rate and a lower feed efficiency are seen when aflatoxin is consumed in feed at levels between 100 and 400 ppb. Young animals and breeding stock are most sensitive to aflatoxicosis. Liver damage, bleeding disorders, and death may occur when aflatoxin levels exceed 400 ppb. At these levels, sows may abort or farrow dead pigs.
Aflatoxins are a major concern to milk producers since the FDA has established an action level of 0.5 ppb aflatoxin M1 in milk. To help lower the probability of contaminated milk, the FDA has established an action level of 20 ppb aflatoxin in dairy feed. Beef cattle are somewhat less susceptible to aflatoxin.
Aflatoxins may increase stress susceptibility and compromise growth efficiency. Chronic symptoms include liver damage, reduced growth, decreased feed efficiency, kidney damage, anemia, interference with the immune system, greater susceptibility to bruising, and interference with normal protein and fat metabolism. Signs of acute aflatoxicosis include depression, nervousness, abdominal pain, diarrhea, rectal prolapse,
and death.
Government Regulations: In the United States, aflatoxin is considered an unavoidable contaminant in food and feed where Good Manufacturing Practices (GMPs) have been followed. The FDA has established guidelines for acceptable levels of aflatoxins in food and feed (Table 1). These guidelines are referred to as action levels, which means that grain, food, or feed exceeding these levels are subject to removal from interstate commerce.
Reconditioning Strategies: Corn containing actionable levels of aflatoxin may be reconditioned. The FDA will permit reconditioning of aflatoxin-contaminated corn lots at export locations by mechanical cleaning with the following conditions:
1. Only one attempt at reconditioning is allowed and the analytical results from the reconditioned lot will be the final determination for disposal of the entire lot.
2. To assure proper reconditioning, the grain company must mechanically clean the lot at a cleaning rate not to exceed 50 percent of the rated cleaner capacity.
3. The Federal Grain Inspection Service (FGIS) must oversee the cleaning process and evaluate the reconditioned corn.
4. FGIS must evaluate the cleanings or screenings for aflatoxin contamination.
The FDA may modify the above reconditioning procedures for domestic corn lots to provide for a cost-effective process. The disposal of corn and screenings resulting from the reconditioning process occurs as follows:
– Cleanings or screenings may be used for animal feed if the aflatoxin content meets FDA feed guidelines.
– Reconditioned corn with less than 20 ppb aflatoxin may be handled without restrictions. If the corn continues to exceed the 20 ppb level, disposal is based on current FDA policy.
Reconditioning corn lots using mechanical cleaners is allowed under the premise that much of the aflatoxin resides on broken corn, which is more susceptible to fungal invasion and toxin production. There are no other FDA approved strategies for decontaminating corn.
Detection: Aflatoxin and other mycotoxins can be measured using relatively simple and inexpensive technology. The black light method only works for aflatoxin and is a presumptive (not a definitive) test. This technology is based on the use of ultraviolet light to produce a bright greenish-yellow fluorescence from kojic acid. This acid is produced by the same fungi that produces aflatoxin, thus, it indirectly indicates the potential presence of aflatoxins. The black light method is no longer used by FGIS because of the high probability of false positives and negatives.
Serological (immunodiagnostic) methods for detecting aflatoxin and other mycotoxins are available through a number of detection kits that are fairly quick, inexpensive, and easy to perform. These kits use antibodies that selectively capture a specific mycotoxin that has been extracted from the grain or feed ingredient. After the extract has been applied to the test kit and the mycotoxin captured by the antibody, a color indicator is added. Depending on the kit, the presence or absence of the mycotoxin is indicated by the intensity of color. Some of these kits may be referred to as an ELISA test, which stands for enzymelinked immunosorbent assay.
The use of chromatography, either thin layer, gas, or high performance liquid chromatography, is a more reliable method of mycotoxin detection used by commercial laboratories.
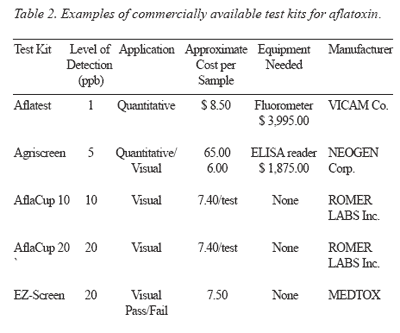
A list of the detection methods for aflatoxins is presented in Table 2.
Sample Collection: The manner in which samples are obtained and processed is an important consideration
when testing for aflatoxin and other mycotoxins. Samples of grain must be representative of the lot and of sufficient size to compensate for the uneven distribution of the contaminant as well as the ultralow levels (parts per billion) that must be detected. Variability in grain samples used for mycotoxin analysis occurs because individual kernels do not contain the same amount of the contaminant, not all kernels are contaminated, and their distribution throughout the load may not be uniform.
FGIS recommends a minimum of 2 pounds of sample be collected from trucks, 3 pounds from railcars, and 10 pounds from barges. Sampling schemes for collecting grain for mycotoxin detection are the same as those used by FGIS for collecting a representative grain sample for grading purposes, described in bulletin MF-2036.
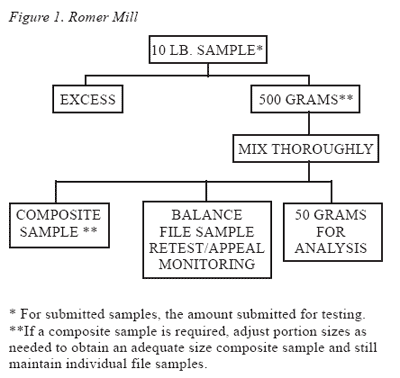
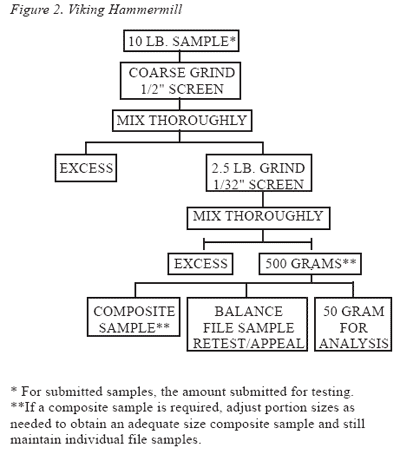
The entire sample should be ground and then subsampled using either of the schemes depicted in Figure 1 or 2. Two types of grinding apparatus used in these flow diagrams are the Romer Mill (Figure 1) and the Viking Hammermill (Figure 2). Although these procedures were developed for the identification of aflatoxin in corn, their use for sampling and sample preparation are appropriate for other mycotoxins.
Deoxynivalenol and Trichothecenes
Deoxynivalenol (DON), commonly referred to as vomitoxin, is produced by Fusarium species. F. graminearum, a Fusarium species which causes root, stalk, and ear rots of corn and sorghum also causes scab in wheat and produces DON in all of these grains.
Field conditions that favor the invasion of these grain crops include warm, moist weather. Blight symptoms can develop within 3 days after infection when temperatures range between 25ºC and 30ºC (77ºF and 86ºF) and moisture is continuous. Plants appear most susceptible when they are infected at the flowering stage of development.
DON is not known to increase in stored shelled corn or in small grains that come contaminated from the field since Fusarium growth requires a minimum moisture content of 19 to 25 percent.
T-2 toxins also are produced by Fusarium species and may occur under conditions described above.
Clinical Signs of Trichothecenes: DON belongs to a class of mycotoxins referred to as trichothecenes, which also include the mycotoxin identified by the name, T-2. General signs of trichothecene toxicity in animals include weight loss, decreased feed conversion, feed refusal, vomiting, bloody diarrhea, severe dermatitis, hemorrhaging, decreased egg production, abortion, and death.
Swine appear to be most susceptible to DON. Feeds containing more than 1 part per million (ppm) of DON may result in a reduction of feed intake and lower weight gain. Vomiting may occur in some cases. Cattle and poultry appear to be less susceptible to DON.
T-2 toxins in poultry may produce lesions at the edges of the beaks, abnormal feathering, reduced egg production, eggs with thin shells, reduced bodyweight gain, and mortality. Mild enteritis and loose feces may occur in calves consuming feed contaminated with T-2.
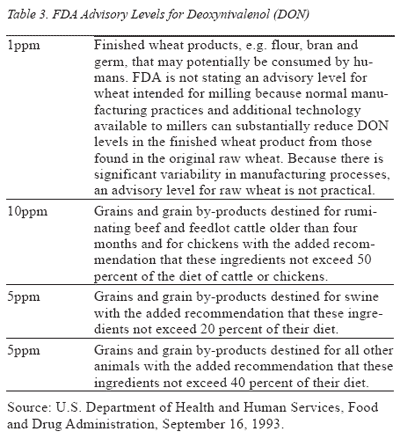
Government Regulations: The FDA has established advisory limits for the presence of DON in food and feed grains. Although these guidelines do not possess the same regulatory authority as the action levels specified for aflatoxin, commercial grain handlers and processors voluntarily follow these FDA recommendations (Table 3).
Reconditioning Strategies: On wheat, cleaning and removing the dockage have been shown to reduce DON concentration by 35 percent. The efficiency of cleaning methods depends on the proportion of total DON present in the shriveled, lighter weight, severely infected kernels with those of normal weight and size. Milling generally partitions Fusarium toxins in milled products. At low levels of fungal invasion, DON and zearalenone are mostly present in the bran and shorts. The flour generally contains the lowest toxin concentration. When the kernels are heavily invaded, toxin levels are usually about the same in milled fractions.
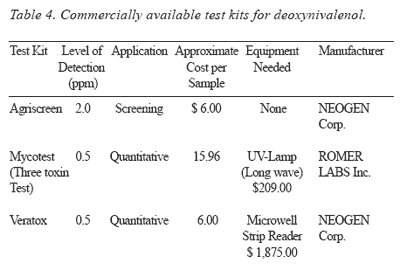
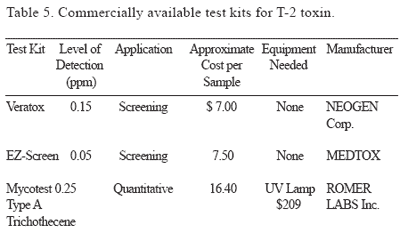
Detection: Commercially available test kits for the detection of DON and T-2 toxins are presented in Tables 4 and 5.
Zearalenone
Zearalenone is typically produced by the fungus Fusarium graminearum; however, other Fusarium species may produce some zearalenone. Corn is the major source of this mycotoxin.
The field conditions described for the production of DON also apply for zearalenone. Exposure to conditions that retain the kernel moisture content between 22 and 25 percent, such as a delay in harvest for several weeks, will favor the growth of the fungus and production of zearalenone. Production of the toxin in storage is unlikely, unless moisture exceeds 22 percent.
Clinical Signs of Zearalenone: Zearalenone is best known for its estrogenic effect in swine. When consumed at levels of a few parts per million, this toxin causes the estrogenic syndrome characterized by a swollen vulva in females and enlarged mammary glands in young males. Zearalenone can cause embryonic death, inhibition of fetal development, and decreased numbers of fetuses present in sows.
Government Regulations: Presently, no advisory limits or action levels have been established by the FDA for zearalenone.
Reconditioning Strategies: See the section under trichothecenes.
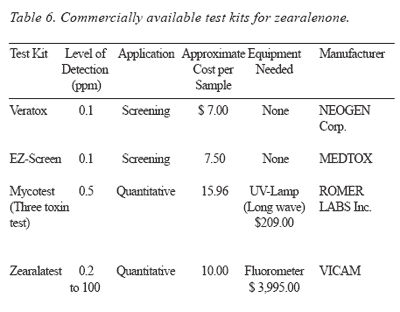
Detection: Commercially available test kits for the detection of zearalenone are presented in Table 6.
Ochratoxin A
Ochratoxins are produced by several species of Aspergillus and Penicillium. They can occur in cereal grains, dry beans, and moldy peanuts. Ochratoxins occur most readily in storage of high-moisture (greater than 22 percent) grains. Therefore, the only recommended control is to keep grains cool and dry in storage.
Clinical Signs of Ochratoxin A: Ochratoxin A can affect changes in the renal function of pigs. Poultry symptoms include retarded growth, decreased feed conversion, impaired kidney function, and mortality. Ochratoxin A can cause a decrease in egg shell quality and egg production.
Government Regulations: Presently, no advisory limits or action levels have been established by the FDA for ochratoxin A.
Reconditioning Strategies: There are different strategies to reduce human exposure to ochratoxin or to reduce its toxic effects when fed to animals. These include prevention of fungal growth in storage, feeding contaminated grain to animals less susceptible to the toxic effects of ochratoxin such as ruminants, and modification of animal diet to promote hydrolysis of ochratoxin.
Moisture content and temperature are the most important factors that affect growth of toxin-producing molds. Generally, fungi do not produce ochratoxin on cereal grains stored at moisture contents lower than 15 percent. Antioxidants such as ascorbic acid have been shown to reduce the toxic effects of ochratoxin A in laying hens.
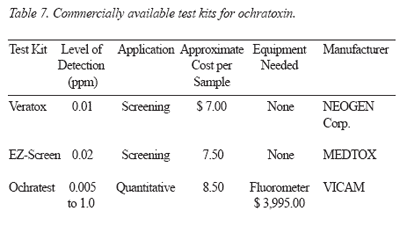
Detection: Commercially available test kits for ochratoxin are presented in Table 7.
Fumonisins
Fumonisins are produced by Fusarium verticillioides, F. proliferatum and other Fusarium species that typically occurs in corn. These fungi infect corn roots, leaves, stalks, and kernels. F. verticillioides survives on crop residue in or on the soil surface and, under favorable conditions, can infect corn stalks directly or through wounds caused by hail or insects. The fungus is commonly seedborne. Fumonisins are reported to occur on visibly healthy grains. Of the currently identified fumonisins, B1, B2, and B3 are the most abundant in naturally contaminated foods and feeds and fumonisin B1 (FB1) generally comprises 75 percent of the total content.
Clinical Signs of Fumonisins: Horses appear to be most susceptible to fumonisin. This mycotoxin, when fed to horses, causes a unique neurotoxic syndrome called leukoencephlomalacia (ELEM). This disorder is characterized by liquefaction of the horse's brain. Neurotoxic symptoms include lowered feed consumption, lameness, oral and facial paralysis, seizures, and eventual death. It has been shown that the toxin is carcinogenic and also associated with pulmonary edema in swine. In humans F. verticillioides has been associated with high rates of esophageal cancer in areas of the world where corn is the main food source such as in the Transkei region in South Africa and Linxian, China.
Government Regulations: Fumonisins have not been regulated by the FDA or USDA. However, the American Association of Veterinary Laboratory Diagnosticians (1992) recommended 5 ppm for horses, 10 ppm for swine, and 50 ppm for cattle and poultry as maximum safe levels of FB1 on feeds.
Detoxification Strategies: There is limited information on detoxification of fumonisins. However, it has been shown that ammoniation reduces fumonisin levels. During ethanol fermentation, no fumonisin could be detected in distilled ethanol, however, fumonisin residues remain in the distillers dried grains. This fermentation product accounts for 31 to 35 percent of the total fumonisin B1 in the starting corn. Thus, ethanol fermentation products generally used as animal feeds could be detrimental if consumed by pigs or horses because these animals are sensitive to fumonisin B1.
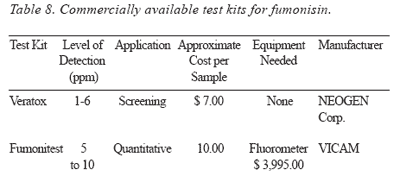
Detection: Presently, methods most commonly used for fumonisins are high performance liquid chromatography and mass spectrometry. Commercially available ELISA-based tests are presented in Table 8.
Summary
In storage, the development of mycotoxins in grain and other feed ingredients can be avoided. This is achieved by preventing the growth of toxin producing molds. Mold growth can be prevented by ensuring moisture and temperature conditions favorable to growth and toxin production do not occur.
The presence of molds in grains and feed ingredients does not automatically mean that toxins are present. Mycotoxins can, however, occur naturally in the field, making it very important to monitor for their presence in feed grains and ingredients. Since mycotoxins are unevenly distributed in commodities, it is important to get a sufficiently representative sample and prepare the sample properly so reliable results are obtained.
Mycotoxins are a concern because they can have chronic and acute effects on animals as well as humans. Aflatoxins and deoxynivalenol are the mycotoxins regulated by the Food and Drug Administration.
References
American Association of Veterinary Laboratory Diagnosticians. 1992. Iowa State University.
MF-2036. 1995. Sampling Feed Components and Finished Feed. KSU Cooperative Extension Service. Manhattan, KS.
CAST 1989. Mycotoxins, Economic and Health Risks. Task Force Report No. 116. Council for Agricultural Science and Technology.
Acknowledgment
Tables provided by Dionisia Teigo-Stockli, Grain Mycologist, formerly with the Department of Grain Science and Industry.
Related topics:
Recommend
Comment
Share
Fast Feed Mills
30 de noviembre de 2008
Informative as well as technical article about Mycotoxins in Feed Grains and Ingredients, good cotribution.
Recommend
Reply
Recommend
Reply

9 de junio de 2008
Good article. But let us be exact and get harmonised on the origin of the word =mycotoxin=. Some people still hold the view that it was coined in 1962 at the peak of the veterinary crisis that hit the UK.Is it then possible that the word =mycotoxicosis= now said to be first used in 1955 ,evolved before the word it was describing? We need to speak with one voice on this issue.
Dr Fapohunda, Stephen
Babcock University, Ilishan, Nigeria
Recommend
Reply
6 de junio de 2008
Very infomative. However further research should be done to determine the actual toxicity caused by mycotoxins and ways to reduced the incidence.
Recommend
Reply
4 de junio de 2008
Thanx, very nice piece of information about Mycotoxins in Feed Grains and Ingredients; more effort to be needed for identification of disease conditions due to moldy corns in livestock, equine, poultry and pig. More work should be carried out for prevention of the effect of these feeds.
Dr Kedar Karki
Nepal
Recommend
Reply

Would you like to discuss another topic? Create a new post to engage with experts in the community.









.jpg&w=3840&q=75)