INTRODUCTION
Advancements in clinical veterinary medicine have resulted in portable diagnostic tools that provide rapid, cost-effective means to investigate metabolic disturbances using blood samples. The handheld i-STATR 1 (2006) analyzer (Abbott Laboratories, East Windsor, NJ) requires a relatively small blood sample (∼100 μL), can be performed “pen-side,” and may be useful in population medicine for commercial egg production systems. The i-STATR 1 has been used for blood gas and chemistry analysis in a variety of animals, including rodents, cattle, exotic avian species, and fish (Tinkley et al., 2006; Rettenmund et al., 2014; Harter et al., 2014; Yildirim et al., 2015). Steinmetz et al. (2007) validated the i-STATR 1 with traditional blood gas and chemistry analyzers for layer chicken blood samples.
Through genetic selection, modern egg laying hens have become highly efficient birds that produce large numbers of eggs. Specialized nutrition programs and advancements in housing and management are critical to achieving genetic potential for optimal egg production performance. Understanding clinical or sub-clinical metabolic derangements related to blood gas, acid-base balance, and electrolytes may provide valuable insight to underlying conditions that affect pullets or laying hens. Establishing blood gas and chemistry reference ranges with a pen-side diagnostic tool for commercial laying hens provides producers, researchers, and veterinary professionals additional diagnostic capabilities when exploring the effects of environment and diet on performance, metabolic disturbances, and disease, and could potentially enhance selection capabilities in pedigree breeding programs by providing physiological measurements for heritable traits. For example, Martin et al. (2011) utilized the i-STATR 1 handheld analyzer to characterize clinical manifestations of calcium disturbances in clinically immobile broiler breeder hens.
Blood gas and chemistry reference ranges for broiler breeder hens have been established previously with the i-STATR 1 device (Martin et al., 2010). Although reference ranges for broiler breeder hens may provide some value when interpreting blood gas and chemistry results for egg layer strains, there are vast differences among broiler and layer genetic lines, management systems, and nutritional requirements that justify specific focus. In the current study, Hy-Line W-36 pullets and hens housed in commercial Midwest facilities were sampled to determine variety-specific venous blood gas and chemistry reference ranges. Focus was placed on collecting samples representing age ranges of significant physiologic phases including pullet development, reproductive maturation during the first laying cycle, and post molt (second cycle) egg production.
MATERIALS AND METHODS
Bird Husbandry
Birds were handled according to company animal welfare policy approved by the veterinarian on staff and all animal procedures were approved by the Institutional Animal Care and Use Committee of Iowa State University before the initiation of experiments. A total of 632 commercial Hy-Line W-36 pullets (at 4, 7, 12, and 15 wk of age; n = 76), first cycle laying hens (at 20, 22, 24, 26, 37, 40, 44, 50, 53, 56, 58, 59, 63, 66, and 68 wk of age; n = 377), and second cycle hens (at 70, 72, 79, 86, 87, 94, 101, 103, and 110 wk of age; n = 179) were sampled across a 7-month time span at 2 fully integrated, multi-age caged layer complexes with caged pullet growing facilities under common ownership, diet formulation, and management systems located in the state of Iowa. At each sampling, approximately 20 birds were selected at random as representative of the entire age matched flock.
Blood Collection and Analysis
Approximately 0.2 to 0.5 mL of blood was collected from the brachial vein with no anticoagulant into a disposable 1 cc syringe. Samples were evaluated with the handheld i-STATR 1 analyzer (Abbot Laboratories) and CG8+ cartridges (Abbott Laboratories), which were kept refrigerated until transported and stored on ice in coolers on the farm. The CG8+ cartridges provide values for sodium (Na mmol/L), potassium (K mmol/L), ionized calcium (iCa mmol/L), glucose (Glu mg/dl), hematocrit (Hct% Packed Cell Volume [PCV]), pH, partial pressure carbon dioxide (PCO2 mm Hg), partial pressure oxygen (PO2 mm Hg), total concentration carbon dioxide (TCO2 mmol/L), bicarbonate (HCO3 mmol/L), base excess (BE mmol/L), oxygen saturation (sO2%), and hemoglobin (Hb g/dl). Blood samples were tested immediately after collection. The needle was removed and a few drops of blood were discarded before dispensing one drop of whole blood to the CG8+ cartridge, sealing the cartridge port, and inserting the cartridge into the hand-held unit (iSTATR 1 operating manual). Performance of the iSTAT instrument was verified daily as specified by the manufacturer. The instrument will not function outside the manufacturer’s specified temperature range. If one or more values did not register on a cartridge for a given sample, the entire data set was not utilized in the analysis.
Statistical Analysis
In order to determine statistical differences among each sample group, the data were analyzed using one-way analysis of variance followed by a t-test using PROC GLM in SAS (2010). Data were investigated comparing pullets, first cycle laying hens, and second cycle laying hens. Relationships between the measured traits were evaluated using linear (Pearson) correlation coefficient of PROC COR in SAS. The spectral decomposition of the correlation matrix was performed. This procedure factors the correlation matrix and simplifies the (co) variance structure, thus representing the variation in the data set in terms of (1) eigenvalues (or factorized proportion of the total variation in the data set), and (2) eigenvectors (with one vector for each eigenvalue, representing direction and magnitude of variation for each variable in the set). The resulting principal component analysis is presented. Age-related variables were evaluated using PROC GLM in SAS.
RESULTS AND DISCUSSION
Pen-side diagnostic tools adapted from clinical human and veterinary medicine provide alternative testing mechanisms for production animal systems such as egg layer complexes. Points of care analyzers, such as the handheld i-STATR 1 analyzer from Abbot Laboratories, allow for on-farm, real-time examination of blood samples, which may assist in understanding pullet or laying hen physiology on an individual bird or flock basis. Such diagnostic tools require reference ranges for accurate interpretation of results and, to the authors’ knowledge, the existing literature does not address blood gas and chemistry of laying hens specifically. In the current investigation, clinically normal commercial Hy-Line W-36 pullets and laying hens were sampled to produce reference ranges for future comparison in disease investigation or potential use in breeding program selection criteria.
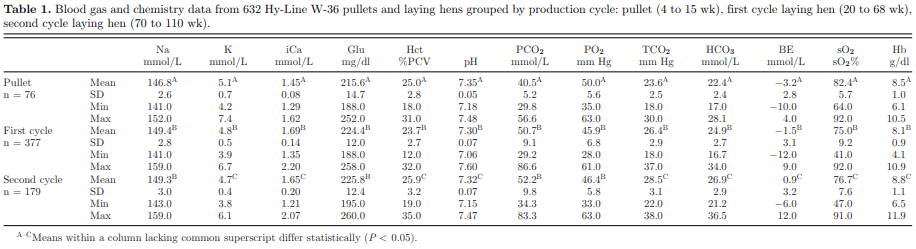
The effects of laying hen age on blood gas and chemistry data are outlined in Table 1. Mean values for all parameters measured on the i-STAT R 1 analyzer and CG8+ cartridge (Na, K, iCa, Glu, Hct, pH, PCO 2, PO 2 , TCO 2, HCO 3, BE, sO 2, Hb) were significantly different between pullets and first cycle layers, as well as pullets and second cycle laying hens. Means for K, iCa, Glu, Hct, pH, TCO 2, HCO 3, BE, sO 2 and Hb differed significantly between first cycle and second cycle layers. There were statistically significant differences observed among the Hct, pH, TCO 2, HCO 3, BE, sO 2, and Hb comparing pullet, first cycle, and second cycle laying hen blood samples. Many of these parameters are tightly interconnected and minor alterations likely reflect the ability of the birds to maintain homeostatic regulation of acid base balance, blood volume, and oxygenation of tissues. Increase in venous PCO2 and decrease in PO2 with age may reflect the requirements of tissue metabolism expected in a growing and mature animal but also may be explained by metabolic differences or environmental effects at the time of sampling. Increased dietary bicarbonate supplementation may contribute to a shift toward metabolic alkalosis and trigger respiratory compensation via an increase in pCO 2 (Davison and Wideman, 1992).
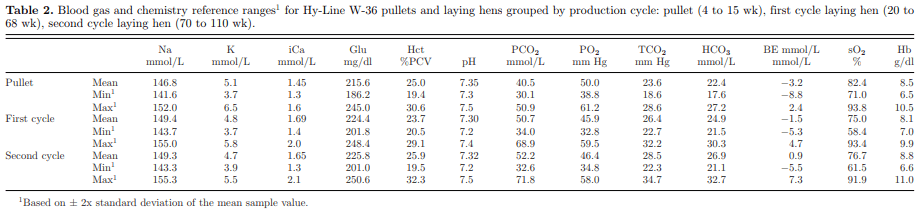
Reference ranges for pullet, first cycle, and second cycle laying hens using the i-STAT R 1 analyzer and CG8+ cartridge are shown in Table 2, accounting for 2 standard deviations from the mean value for each trait. These reference ranges were determined from multiple hens of multiple ages but all of the same commercial variety—the Hy-Line W-36. It is likely that other commercial White Leghorn varieties would have similar values. Brown egg layers are produced from different breeds. Reference ranges for brown egg layers are currently being obtained.
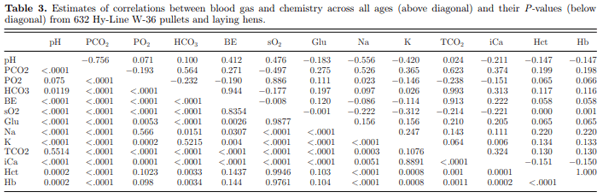
Many of the blood chemistry parameters were significantly correlated (Table 3). Some of the correlation coefficient estimates exceeded a value of 0.9, which suggests that one of each pair of the values could be removed from the panel without substantial loss of information. For instance, BE, TCO2, and HCO3 had pair-wise correlations >0.9. These correlations are anticipated as these 3 traits are indicators of the metabolic component of acid-base status and are calculated according to the measured values of pH and PCO2 in a particular sample (Montesinos and Ardiaca, 2013; iSTATR 1 System Manual, 2006). In addition, Hb and Hct showed a correlation of 1.0 since Hb is calculated directly from Hct. A closer inspection of many of the pair-wise correlations may help in better understanding physiologic factors expressed in blood chemistry as presented by the iSTATR 1 device, when using the CG8+ cartridge.
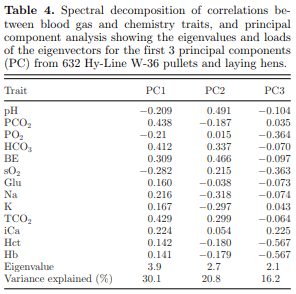
Another possible way to summarize and interpret relationships among multiple blood gas and chemistry indicators is by implementing spectral decomposition of the correlations presented in Table 3. When this was done, a principal component (PC) analysis (Table 4 ) indicated that the first 3 eigenvalues explained ∼2/3 (67.03%) of the total variation in the data; furthermore, the first 2 PC explained more than half (50.9%) of the total variation. The corresponding loads in the PC1 eigenvector showed positive values for all the parameters, dominated by CO2 (0.44), TCO2 (0.43), HCO3 (0.41), and BE (0.31); while the only parameters with negative loads were pH (−0.21), PO2 (−0.21), and sO2 (−0.28). The second PC loads were mainly dominated by pH (0.49) and BE (0.47). This clearly summarizes the corresponding correlation values and indicates the importance of the acid-base balance and buffer factors, and the relationship between blood oxygen and CO2 in maintaining overall system homeostasis.
The third PC explained 16% of the total variation and, interestingly, positive loads were dominated by iCa (0.23). The other third PC positive loads are negligible while showing negative loads for other blood chemistry and gas values, which reflects an increase in calcium at the expense of other components. This analysis provides insight into the relative importance of calcium in blood chemistry, and its influence on other blood chemistry components in layer chickens. This is not unanticipated, as calcium is the key component in egg formation and its carbonate salt makes up about 96% of the total eggshell (Hincke et al., 2012). Dynamic blood calcium metabolism is required not only for intestinal absorption from dietary sources but also calcium mobilization from medullary bone to supplement the needs for eggshell formation (Campbell, 2012).
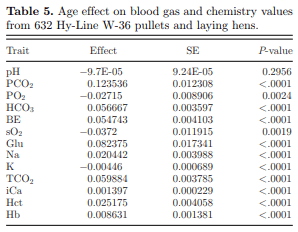
Except for pH, age expressed in wk had a significant linear relationship with all analyzed blood gas and chemistry traits as outlined in Table 5. This underscores the importance of maintaining blood pH at all ages to ensure bird system homeostasis. All blood gas and chemistry values measured on the CG8+ cartridge (except PO2, sO2, and K) tended to increase with age.
Differences between blood gas and chemistry values for pullet and mature laying hens in both the first and second laying cycle may be explained by the effects of reproductive maturation and subsequent hormonal regulation for egg production. Additionally, diet between layer pullets and laying hens differ in nutrient content, which may ultimately affect blood chemistry values (Hy-Line, 2015). Diet supplementation of Na (via NaCl or NaHCO3) can increase Na systemically and may explain the significant difference among Na levels in pullets compared with first cycle and second cycle layers (Hochleithner, 1994). Potassium levels in second cycle layers were found to be significantly lower than samples from first cycle layers and pullets. One explanation may be related to water consumption, which is often inversely related to body size (Campbell, 2012). Relatively decreased water consumption in adult birds can induce the release of aldosterone via the renin-angiotensin-aldosterone system, which acts to resorb Na and water from the kidneys in exchange for K. Ionized calcium levels differed significantly among all age groups, and were highest in the first cycle hens and lowest in pullet blood samples. This is explained by dietary calcium and metabolic differences mobilization of skeletal calcium for eggshell production. Pullet rations contain the lowest calcium levels, followed by laying hen rations, and the second cycle hen rations contain the highest calcium percentage in the layer diet (Hy-Line, 2015).
Blood chemistry and blood gas reference data for pullets and laying hens may be used to tailor nutrition programs and bird husbandry techniques, especially in cases of stress (temperature extremes, vaccination, or disease). Olanrewaju et al. (2006) have reported alter in ations in blood PO2, PCO2, Na, K, Cl, Glu, and anion gap in broiler chickens given continuous infusion of adrenocorticotropic hormone over 14 d as a model for simulating stress. Alternatively, data may provide selection information for individual birds or populations in breeding programs. For example, the effects of heat stress on blood pH, calcium metabolism, egg quality, and egg production are documented (Persia et al., 2003). Addressing bird stress (heat exposure, disease, etc.) is critical to achieving genetic potential of egg laying hens; however, subclinical stress may not overtly impact visible production factors. Use of the i-STATR 1 analyzer may provide valuable information for resolving bird issues before production traits are obviously impacted.
Unit cost for the i-STATR 1 analyzer (current list price $11,500 USD) and functionality in a farm setting are challenges that can be improved upon to produce a more cost-effective, reliable tool for use in production medicine. The current list price for the single use CG8+ cartridges is $15.12 each or $378 per box of 25 cartridges, which presents an additional expense per use. Unfortunately, cartridge errors occurred at a rate of 5 to 50% during sample analyses. Errors were likely a result of dust, barn temperature, clotted blood samples, air bubbles in the cartridge, or incorrect cartridge filling technique. Additionally, the i-STATR 1 handheld unit produced error codes due to hot or cool ambient barn temperatures, which are less likely to occur in a clinical setting. Use of an anticoagulant, such as lithium heparin, for poultry samples as described by Martin et al. (2010), along with prompt handling of samples in an air-conditioned space, would likely reduce the error rate.
This article was originally published in 2016 Poultry Science 95:466–471 http://dx.doi.org/10.3382/ps/pev350. This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/bync/4.0/). 















.jpg&w=3840&q=75)