This article was originally published in British Poultry Science (2015), Vol. 56, No. 6, 723–732.
INTRODUCTION
Concerns regarding development of antibioticresistant microorganisms and social pressures have continued the trend to ban the use of antibiotics as growth promoters in poultry production (Castanon, 2007). This has also resulted in an urgent necessity to find feasible alternatives to maintain poultry health, in order to sustain poultry as an economically viable source of animal protein for human consumption (Alvarez-Olmos and Oberhelman, 2001). In this regard, the use of selected strains from different beneficial microorganisms from the genus Bacillus and Lactobacillus have shown to be a suitable option for the poultry industry (Tellez et al., 2012). Bacillus spp. are gram-positive, facultative aerobe, endospore-forming, rod-shaped bacteria normally found in soil and water sources, as well as in the gastrointestinal tract of animals and humans (Hong et al., 2009).
Its multiple flagella allows it to move quickly in liquids. Bacillus spp. are the most investigated gram-positive bacteria and a model organism to study bacterial chromosome replication and cell differentiation and together with other beneficial microbes have been extensively used as a source of industrial enzymes and antibiotics by biotechnology companies (Hendricks et al., 1995; Kunst et al., 1997; Monisha et al., 2009). When environmental conditions are not favourable for growth and replication of bacteria from the genus Bacillus, dramatic metabolic changes occur, such as the induction of chemotaxis, cannibalism, production of macromolecular hydrolases (proteases and carbohydrases), as well as the formation of endospores (González-Pastor et al., 2003; Hong et al., 2005; López et al., 2009; Higgins and Dworkin, 2012). Due to the capacity of bacterial spores to resist harsh environmental conditions and long storage periods, endospores from selected Bacillus strains have been used as reliable direct-fed microbials (DFM) in animal production (Tellez et al., 2013). Additionally, Bacillus-DFM have previously been shown to prevent gastrointestinal disorders and impart numerous nutritional benefits for animals and humans (Duc Le et al., 2004; Cartman et al., 2007; Sen et al., 2012). Recent studies published by our laboratory have shown that approximately 90% of B. subtilis spores germinate within 60 min in presence of feed in vitro and in vivo in different segments of the gastrointestinal tract (Latorre et al., 2014a). After spore germination into vegetative cells, Bacillus spp. bacteria become metabolically active to produce chemical compounds that are beneficial to the host and the intestinal microflora (Jadamus et al., 2001; Leser et al., 2008).
In most of the United States and in other countries, including Brazil, broiler feed is based primarily on maize and soybean meal, which supplies the majority of energy and protein in the diet. Utilisation of the nutrients contained in maize by broilers is generally considered to be high. Nevertheless, at times it is difficult to formulate least cost diets using maize and unconventional grains with variable concentrations of anti-nutritional factors have to be used. Rye (Secale cereale) is a cereal member of the wheat tribe (Triticeae) and has been reported to contain 152 g of total non-starch polysaccharides (NSP) per kg of dry matter (Antoniou et al., 1981; Bach Knudsen, 1997). When chickens are fed on alternative cereal grains such as rye that are high in soluble NSP, high digesta viscosity, poor nutrient digestibility and reduced bone mineralisation have been reported, resulting in decreased growth performance and reduced litter quality conditions caused by sticky droppings (Campbell et al., 1983; Fengler and Marquardt, 1988). However, different studies have shown that the inclusion of carbohydrases such as xylanase in rye-based diets significantly improved all these negative factors reducing the impact of the anti-nutritional components present in the rye grain (Bedford and Classen, 1993; Dänicke et al., 1997; Silva and Smithard, 2002). Previously, we have evaluated the inclusion of selected Bacillus-DFM candidates that produce a different set of extracellular enzymes using different poultry diets in vitro (rye, wheat, barley and oat based-diets), resulting in a significant reduction in both digesta viscosity and Clostridium perfringens proliferation between control diets and Bacillus-DFM supplemented diets (Latorre et al., 2015). The objective of the present study was to evaluate the role of a multiple enzyme producing Bacillus-based DFM on growth performance, digesta viscosity, bacterial translocation, microbiota composition and bone mineralisation in broiler chickens fed on a rye-based diet.
MATERIALS AND METHODS
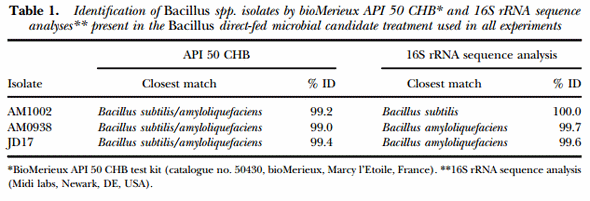
Isolation and characterisation of Bacillus spp. Previous research conducted in our laboratory focused on isolation of several Bacillus spp. from environmental and poultry sources (Shivaramaiah et al., 2011; Wolfenden et al., 2011; Menconi et al., 2013). Identification and characterisation of the different isolates was carried out using a bioMerieux API 50 CHB test kit (catalogue no. 50430, bioMerieux, Marcy l’Etoile, France) and individual plates of each strain were also sent for 16S rRNA sequence analysis to a specialised laboratory (Midi labs, Newark, DE, USA). One of the three Bacillus strains (AM1002) was identified as B. subtilis and the other two isolates (AM0938 and JD17) were identified as B. amyloliquefaciens (Table 1). These Bacillus strains were selected as superior producers of cellulase and xylanase based on a qualitative enzyme activity evaluation performed using a different selective media for each evaluated enzyme (Latorre et al., 2014b, 2015). Then, the three Bacillus spp. selected strains were sporulated and mixed during the DFM-candidate preparation process before dietary inclusion.
DFM preparation In an effort to grow high numbers of viable spores, a solid-state fermentation media (SS) developed by Zhao et al. (2008) was selected and modified for use in these experiments. Briefly to prepare the SS fermentation media, ammonia broth was added to a mixture of 70% rice straw and 30% wheat bran at a rate of 40% by weight. Then, the SS fermentation media was added to 250 ml Erlenmeyer flasks and sterilised by autoclaving for 30 min at 121°C. Each of the three Bacillus spp. isolates was grown individually overnight at 37°C in test tubes containing 10 ml of tryptic soy broth (TSB, catalogue no. 211822, Becton Dickinson, Sparks, MD). Following incubation, 2 ml of each isolate culture were added separately to the previously prepared SS fermentation media flask. The inoculated flasks were incubated for 24 h at 37°C to promote growth of the Bacillus spp. vegetative cells and then incubated for another 72 h at 30°C to trigger the initiation of the sporulation process. Following this, the inoculated SS fermentation media was removed from the Erlenmeyer flasks, placed onto petri dishes and dried at 60°C. Then, the SS fermentation media was aseptically ground into a fine powder that contained stable Bacillus spores (~1011 spores/g). Bacillus spp. spores from each of the three selected strains were combined in equal amounts to conform the Bacillus-DFM candidate treatment. Next, the DFM was included into the feed to reach a concentration of 106 spores per g of feed using a rotary mixer for 15 min. Samples of feed containing the Bacillus-DFM candidate were taken to validate the amount of spores per g of feed after the inclusion and mixing steps, a 1:10 dilution was made with saline in glass sterile tubes and all feed samples were incubated at 100° C for 10 min to eliminate the presence of vegetative cells present in the feed allowing the enumeration of spores only. Following heat treatment, 1:10 dilution of the feed samples from the glass tubes were plated on tryptic soy agar plates (TSA, catalogue no. 211822, Becton Dickinson, Sparks, MD); letting spores in the feed sample germinate into vegetative cells after incubation at 37°C for 24 h, hence representing the number of spores present per g of feed.
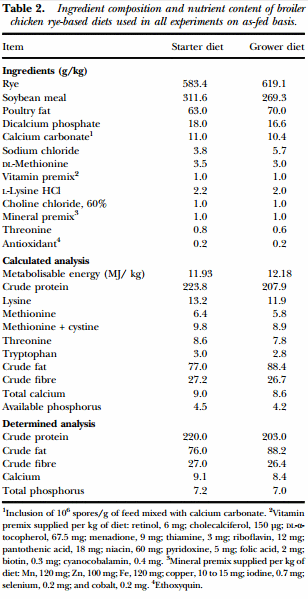
Animal source and diets In the present study, three independent experiments were conducted. For all experiments d-ofhatch male broiler chicks were obtained from Cobb-Vantress (Siloam Springs, AR, USA). In Experiments 1 and 2, chicks were placed in isolation chambers (90 cm × 80 cm) with a controlled age-appropriate environment. Meanwhile in Experiment 3, birds were necktagged and randomly located to one of 16 floor pens (300 cm × 150 cm) with new pine shavings as litter in an environmentally controlled room. Temperature was maintained at 34°C for the first 5 d and was then gradually reduced according to normal management practices, until a temperature of 23°C was achieved at d 21 of age. In all experiments, broiler chicks were randomly assigned to either a control group (CON) consuming a mash rye-based diet or a treated group (TRT) fed on a mash rye-based diet supplemented with the Bacillus-DFM candidate (106 spores/g of feed). In Experiments 1 and 2, a starter diet was fed throughout the experimental period (0 to 10 d). However, in Experiment 3 due to a prolonged duration, starter (0 to 7 d) and grower (8 to 28 d) diets were offered. Prior to formulating the experimental diets, it was determined that the rye grain contained: moisture 109 g/kg, crude protein 124 g/kg, crude fat 18 g/kg, crude fibre 29 g/kg, calcium 0.9 g/kg and phosphorus 3.1 g/ kg. The experimental diets were formulated to approximate the nutritional requirements of broiler chickens as recommended by the National Research Council (1994) and adjusted to breeder’s recommendations (Cobb-Vantress Inc., 2013). No antibiotics or coccidiostats were added to the feed (Table 2). The chemical composition of the experimental diets was determined by AOAC International (2000) methods for moisture (930.15), crude protein (984.13), crude fat (920.39), crude fibre (978.10), calcium (968.08) and phosphorus (965.17). All animal handling procedures were in compliance with the Institutional Animal Care and Use Committee at the University of Arkansas, Fayetteville, USA.
Experimental design Experiments 1 and 2 In order to show that similar results can be achieved independently, two experiments were conducted in the present study in broiler chickens that were raised for 10 d. In each experiment, 50 d-of-hatch, chickens were randomly assigned to one of two groups: rye-based diet or a rye-based diet supplemented with Bacillus-DFM (n = 25/group); the number of animals used was based on published studies in which similar variables were measured (Bedford et al., 1991; Tellez et al., 2014; Latorre et al., 2014b). At 10 d-of-age, in both experiments, all chickens were weighed and humanely killed by CO2 asphyxiation. Samples were obtained from randomly selected individual broilers and analysed separately. The right half of the liver was aseptically removed to evaluate bacterial translocation (n = 12/group). Additionally, digesta samples were taken to evaluate viscosity (n = 8/group) and both tibias were removed to analyse bone quality characteristics (n = 12/group). Moreover, in both experiments duodenal, ileal and caecal gut sections were obtained to enumerate different bacterial populations (n = 12/ group). Details about measurement techniques are described below.
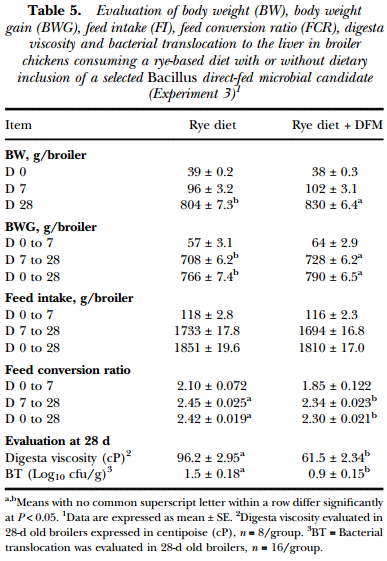
Experiment 3 As an extension of Experiments 1 and 2 and for evaluation of the rye-based diet during the starter and grower production periods, 320 d-of-hatch chickens were neck tagged and randomly allotted based on initial body weight (BW) to one of two groups; control rye-based diet or Bacillus-DFM supplemented rye-based diet. Each treatment was comprised of 8 replicates of 20 chicks each (n = 160/group) and for evaluation of growth performance, each replicate was used as experimental unit. Weekly, all broilers were individually weighed and BW, body weight gain (BWG) and pen feed intake (FI) were noted at the end of each phase to calculate the feed conversion ratio (FCR) for starter (0 to 7 d), grower (8 to 28 d) and overall (0 to 28 d) experimental phases. At 28 d-of-age, all chickens were weighed and humanely killed by CO2 asphyxiation. Samples were obtained from two randomly selected broilers for bacterial translocation and bone quality determination (n = 16/group). In the case of evaluation of digesta viscosity, one bird per replicate was selected to collect the intestinal content (n = 8/ group). Details about measurement techniques are described below.
Digesta viscosity Total intestinal contents were obtained from duodenum to cloaca in Experiments 1 and 2 to evaluate digesta viscosity. In Experiment 3, digesta were taken from duodenum to Meckel’s diverticulum. Approximately 1.5 g (wet weight) of the fresh digesta were immediately centrifuged (12000 g) for 5 min. The supernatant was obtained and stored on ice until viscosity was determined using a LVDV-I Brookfield digital cone-plate viscometer fitted with a CP-40 spindle (Brookfield Engineering, Middleboro, MA). The analysed samples and the viscometer cup were maintained at a temperature of 40°C during viscosity measurements to simulate broiler’s body temperature conditions. Viscosity was measured in centipoise (cP = 1/100 dyne s/cm2 ).
Bacterial translocation Briefly, the right half of the liver was removed from each chicken, collected into sterile bags, homogenised, weighed and 1:4 w/v dilutions were made with sterile 0.9% saline. Then, 10-fold dilutions of each sample were made in a sterile 96 well Bacti flat bottom plate and the diluted samples were plated on MacConkey Agar (VWR Cat. No. 89429–342 Suwanee, GA 30024). Biochemical evaluation tests as well as identification of isolated colonies that grew on the MacConkey agar plates were carried out using a bioMerieux API-20E test kit (catalogue no. 20100, bioMerieux, Marcy l’Etoile, France). Bacterial translocation was expressed in colony forming units (log10 cfu/g of tissue).
Intestinal microflora Whole duodenum, ileum and caeca were aseptically removed, separated into sterile bags and homogenised. Samples were weighed and 1:4 w/ v dilutions were made with sterile 0.9% saline. Then, 10-fold dilutions of each sample from each group were made in a sterile 96 well Bacti flat bottom plate and the diluted samples were plated on three different culture media; for enumeration of total recovered lactic acid bacteria (LAB) on de Man Rogosa Sharpe agar (Difco™ Lactobacilli MRS Agar VWR Cat. No. 90004–084 Suwanee, GA 30024); total recovered gram-negative bacteria (TGB) on MacConkey agar and total recovered anaerobes (TAB) on tryptic soy agar plates containing sodium thioglycollate (catalogue no. 212081, Becton Dickinson, Sparks, MD). Bacteria enumeration was expressed in colony forming units (log10 cfu/g of tissue) and all plates were incubated during 18 h at 37°C before bacterial count.
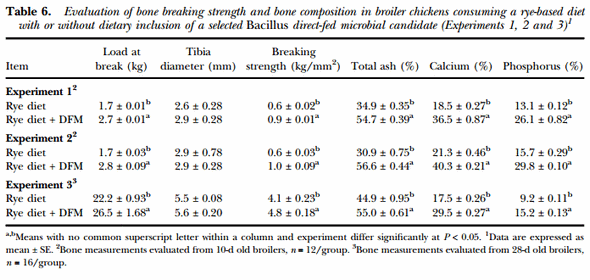
Bone quality Bone quality measurements were made according to the methods described by Zhang and Coon (1997). Tibias from each chicken were cleaned of adherent tissues. Bones from the left leg were subjected to conventional bone assays as described below and tibias from the right legs were used to determine breaking strength. Bones from the left tibia were dried at 100°C for 24 h and weighed. Then the samples were ashed in a muffle furnace (Isotemp muffle furnace, Fisher Scientific, Pittsburgh, PA) at 600°C for 24 h in crucibles, cooled in a desiccator and weighed. From the left tibia, total calcium content was obtained by inductively coupled plasma determination (968.08; AOAC International, 2000) and total phosphorus content was determined by colorimetry using the molybdo-vanadate method (965.17; AOAC International, 2000). In the case of the right tibia samples, the tibial diaphysis from individual birds were cleaned of adherent tissues, the periosteum was removed and the biomechanical strength of each bone was measured using an Instron 4502 material testing machine (Norwood, MA) with a 509 kg load cell. The bones were held in identical positions and the mid-diaphyseal diameter of the tibial mid-shaft, which was also the site of impact, was measured using a dial caliper. The maximum load at failure was determined in the tibial mid-section between epiphyses, using a three-point flexural bend fixture with a total distance of 30 mm between the two lower supporting ends. The load, defined as force in kg per square mm of cross-sectional area (kg/mm2 ), represents bone strength. The rate of loading was kept constant at 20 mm/min collecting 10 data points per second. The data were automatically calculated using Instron’s Series IX Software (Norwood, MA).
Statistical analysis In all experiments, data were subjected to one-way ANOVA as a completely randomised design using the GLM procedure of SAS (SAS Institute, 2002). In Experiments 1 and 2, each measurement obtained from individual broilers from each experimental group was considered as the experimental unit for BW (n = 25/group), digesta viscosity (n = 8/group), liver bacterial translocation (n = 12/group), microbiota composition (n = 12/group) and bone quality parameters (n = 12/group). Additionally, as an extension of Experiments 1 and 2, in Experiment 3 for the evaluation of growth performance (BW, BWG, FI and FCR), each of the 8 replicates of 20 chickens was considered as the experimental unit, whereas data on digesta viscosity (n = 8/group), bacterial translocation (n = 16/group) and bone quality (n = 16/group) were based on randomly selected broilers from all replicates of each group. Data are expressed as mean ± SE and a P-value of P < 0.05 was set as the standard for significance.
RESULTS
The results of the evaluation of BW, digesta viscosity and liver bacterial translocation in broiler chickens consuming a rye-based diet with or without dietary inclusion of a selected Bacillus-DFM candidate in Experiments 1 and 2 are summarised in Table 3. Chickens that received the rye-based diet supplemented with the DFM had a significant increase in BW at d 10-of-age compared to the control group (P < 0.05) and also showed a significant reduction in digesta viscosity and bacterial translocation in both experiments (Table 3). Identification of the gram-negative, lactose positive bacteria translocated to the liver in all experiments was confirmed to be Escherichia coli using the bioMerieux API-20E test kit.
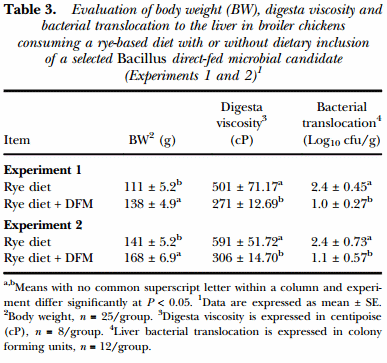
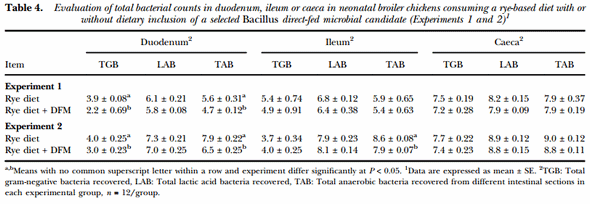
Table 4 summarises the results of the evaluation of total bacterial counts recovered from duodenum, ileum and caeca in 10-d old broiler chickens from Experiments 1 and 2. In both trials, DFM-supplemented chickens had a significant decrease in the number of TGB recovered from the duodenum when compared to the control group. Similarly, in both experiments, a reduction in the number of TAB from the duodenum of chickens consuming the Bacillus-DFM was observed in comparison to the unsupplemented group. Additionally, a reduction in the number of TAB was also showed in the ileum from the DFM group in Experiment 2 (P < 0.05). Nevertheless, similar amounts of LAB were recovered from each intestinal section evaluated in both experiments from both experimental groups. Moreover, comparable bacterial counts were observed between experimental groups in the caeca in both trials for total gram-negative, lactic acid and anaerobic recovered bacteria.
In Experiment 3, during the starter phase (0 to 7d), broilers consuming the diet supplemented with Bacillus-DFM showed similar values in all the growth performance variables (BW, BWG, FI and FCR) that were evaluated in comparison to the control group. On the other hand, during the grower phase (8 to 28d) and the overall study period (0 to 28d), broilers consuming the BacillusDFM candidates had a significantly higher BW and BWG coupled with a more efficient FCR compared to the control group (P < 0.05). Feed intake was similar between both experimental groups throughout the study. In the case of digesta viscosity and bacterial translocation, inclusion of the Bacillus-DFM in the rye-based diet resulted in a significant reduction of viscosity in the intestinal content, together with a decrease in the number of gram-negative, lactose positive bacteria present in the liver, which was confirmed to be Escherichia coli (Table 5).
The effects of the dietary inclusion of the Bacillus-DFM candidate on bone quality in chickens consuming a rye-based diet are shown in Table 6. In Experiments 1 and 2, bone strength and composition were measured in 10-d old broilers, showing a significant improvement in all bone quality measurements in chickens consuming the Bacillus-DFM in comparison to the control group (P < 0.05). Similar results were also obtained in Experiment 3, where bone quality variables were measured in 28-d old broilers, showing an increase in bone strength and percentage of ash, calcium and phosphorus when the Bacillus-DFM was included in the diet (P < 0.05). However, in all experiments similar tibia diameters were observed between experimental groups.
DISCUSSION
When chickens are fed on diets containing grains such as rye instead of maize, poor performance and detrimental litter conditions caused by sticky droppings occurred (Campbell et al., 1983; Fengler and Marquardt, 1988). Rye has an elevated concentration of highly branched arabinoxylans in comparison to other cereals like wheat or maize (Bach Knudsen, 1997). The presence of soluble NSP from rye in the intestinal lumen increases digesta viscosity affecting nutrient availability and absorption (Bedford and Classen, 1993; Choct et al., 1995). The high concentration of soluble NSP in rye-based diets also have an impact on the intestinal bacterial population, probably as a consequence of the increased digesta viscosity and prolonged feed passage time (Choct et al., 1996; Bedford and Schulze, 1998; Kiarie et al., 2013). Furthermore, utilisation of rye in poultry diets has also been related to malabsorption of lipids, deterioration of bone mineralisation and reduced leg soundness (Macauliffe and Mcginnis, 1971). This negative effect on bone quality could also be related to an elevated digesta viscosity, therefore, enhancing the deconjugation of bile acids by the overgrowth intestinal micro- flora, resulting in a reduction of micelle formation, affecting fat solubilisation and absorption of fat soluble vitamins and minerals (Grammer et al., 1982). Since monogastric animals do not have endogenous enzymes capable of hydrolysing the β-linkages present in soluble NSP, exogenous carbohydrases (xylanase, β-glucanase, β-mannanase, α-galactosidase and pectinase) have been used in poultry diets as feed additives in an attempt to reduce the adverse impact of these anti-nutritional factors (Bedford et al., 1991; Bedford and Classen, 1993; Esteve-Garcia et al., 1997). It has been well-documented that inclusion of xylanase in rye-based diets significantly improve viscosity of digesta supernatant, accelerate feed passage time through the gastrointestinal tract and enhance digestibility of dietary protein and fat sources resulting in an improvement in growth performance (Dänicke et al., 1997; Langhout et al., 1997; Lázaro et al., 2004; Lee, 2014). The results of the present study support previous findings published by our laboratory in turkey poults fed on rye-based diets (Latorre et al., 2014b). Similarly, digesta viscosity was considerably higher in chickens fed on rye-based diets without the DFM when compared to broilers consuming the DFM-supplemented diet, the supernatant being more semisolid than liquid in the control group, suggesting that viscosity alone could be directly responsible for poor performance. The increase in digesta viscosity observed in the control group was also associated with elevated bacterial translocation to the liver and overgrowth of gram-negative and anaerobic bacteria in the duodenal section when compared to chickens that consumed the BacillusDFM diet. These differences could be due to less substrate available for bacterial growth, generating lower intestinal inflammation and translocation of bacteria when the intestinal viscosity was reduced by the inclusion of the DFM candidate, suggesting more absorption of nutrients by the intestinal brush border of supplemented broilers. It has been previously reported that alterations in gut permeability are connected with bacterial translocation in the portal and/or systemic circulation during several types of “leaky gut” syndromes leading to bacterial septicaemia (Ilan, 2012; Seki and Schnabl, 2012). On the other hand, significant improvements in BW, BWG and FCR were observed in chickens consuming the Bacillus-DFM supplemented diet when compared to chickens from the control group, suggesting that the production of enzymes from the combined Bacillus spp. strains used as DFM could increase the absorption of nutrients promoting growth performance and a more efficient FCR in addition to enhancing the physical and bacteriological conditions of the intestinal content. Furthermore, the significant reduction in bone strength and mineralisation generated by consumption of rye-based diets con- firmed previous research from different authors that have shown that the inclusion of rye in poultry diets is associated with malabsorption of minerals and fat-soluble vitamins (Macauliffe et al., 1976; Campbell et al., 1983; Wideman and Prisby, 2013). However, the results from the present study suggest that the reduction of digesta viscosity together with the production of phytase by the Bacillus-DFM candidate could enhance the absorption of nutrients including minerals, hence improving bone strength and bone mineralisation.
In conclusion, the present study showed that chickens fed on rye-based diets have an increased digesta viscosity and bacterial translocation associated with overgrowth of gut microflora, low performance and decreased bone mineralisation. However, this is one of the first studies reporting that these adverse effects caused by the utilisation of rye in poultry diets can be minimised by the inclusion of a selected Bacillus-DFM candidate, thereby enhancing intestinal integrity and absorption of nutrients resulting in an improvement of production performance. Large-scale commercial studies to evaluate the dietary supplementation of different poultry diets with the combination of these Bacillus spp. candidate strains are currently being evaluated.
DISCLOSURE STATEMENT
No potential conflict of interest was reported by the authors.
REFERENCES
ALVAREZ-OLMOS, M.I. & OBERHELMAN, R.A. (2001) Probiotic agents and infectious diseases: a modern perspective on a traditional therapy. Clinical Infectious Diseases, 32: 1567–1576. doi:10.1086/320518
ANTONIOU, T., MARQUARDT, R.R. & CANSFIELD, P.E. (1981) Isolation, partial characterization and antinutritional activity of a factor (Pentosans) in rye grain. Journal of Agricultural and Food Chemistry, 29: 1240–1247. doi:10.1021/jf00108a035
AOAC INTERNATIONAL (2000) Animal feeds, in: HORWAITZ, W. (Ed) Official Methods of Analysis of AOAC International, 17th edn. Vol. 1, pp. 1–54 (Gaithersburg, MD, AOAC International).
BACH KNUDSEN, K.E. (1997) Carbohydrate and lignin contents of plant materials used in animal feeding. Animal Feed Science and Technology, 67: 319–338. doi:10.1016/S0377- 8401(97)00009-6
BEDFORD, M. & CLASSEN, H. (1993) An in vitro assay for prediction of broiler intestinal viscosity and growth when fed ryebased diets in the presence of exogenous enzymes. Poultry Science, 72: 137–143. doi:10.3382/ps.0720137
BEDFORD, M., CLASSEN, H. & CAMPBELL, G. (1991) The effect of pelleting, salt, and pentosanase on the viscosity of intestinal contents and the performance of broilers fed rye. Poultry Science, 70: 1571–1577. doi:10.3382/ ps.0701571
BEDFORD, M. & SCHULZE, H. (1998) Exogenous enzymes for pigs and poultry. Nutrition Research Reviews, 11: 91–114. doi:10.1079/NRR19980007
CAMPBELL, G., CAMPBELL, L. & CLASSEN, H. (1983) Utilisation of rye by chickens: effect of microbial status, diet gamma irradiation and sodium taurocholate supplementation. British Poultry Science, 24: 191–203. doi:10.1080/ 00071668308416730
CARTMAN, S.T., LA RAGIONE, R.M. & WOODWARD, M.J. (2007) Bacterial spore formers as probiotics for poultry. Food Science and Technology Bulletin: Functional Foods, 4: 21–30.
CASTANON, J. (2007) History of the use of antibiotic as growth promoters in European poultry feeds. Poultry Science, 86: 2466–2471. doi:10.3382/ps.2007-00249
CHOCT, M., HUGHES, R.J., TRIMBLE, R.P., ANGKANAPORN, K. & ANNISON, G. (1995) Non-starch polysaccharide-degrading enzymes increase the performance of broiler chickens fed wheat of low apparent metabolizable energy. The Journal of Nutrition, 125: 485–492.
CHOCT, M., HUGHES, R.J. & WANG, J. (1996) Increased small intestinal fermentation is partly responsible for the antinutritive activity of non-starch polysaccharides in chickens. British Poultry Science, 37: 609–621. doi:10.1080/ 00071669608417891
COBB-VANTRESS INC. (2013) Broiler performance and nutrition supplement. Available: http://www.cobb-vantress.com/ docs/default-source/cobb-500-guides/cobb500-broiler-per formance-nutrition-supplement-(english).pdf. Accessed 18 Jan 2014
DÄNICKE, S., SIMON, O., JEROCH, H. & BEDFORD, M. (1997) Interactions between dietary fat type and xylanase supplementation when rye-based diets are fed to broiler chickens 2. Performance, nutrient digestibility and the fat-soluble vitamin status of livers. British Poultry Science, 38: 546–556. doi:10.1080/00071669708418035
DUC LE, H., HONG, H.A., BARBOSA, T.M., HENRIQUES, A.O. & CUTTING, S.M. (2004) Characterization of Bacillus probiotics available for human use. Applied and Environmental Microbiology, 70: 2161–2171. doi:10.1128/AEM.70.4.2161- 2171.2004
ESTEVE-GARCIA, E., BRUFAU, J., PEREZ-VENDRELL, A., MIQUEL, A. & DUVEN, K. (1997) Bioefficacy of enzyme preparations containing beta-glucanase and xylanase activities in broiler diets based on barley or wheat, in combination with flavomycin. Poultry Science, 76: 1728–1737. doi:10.1093/ps/ 76.12.1728
FENGLER, A.I. & MARQUARDT, R.R. (1988) Water-soluble pentosans from rye: II. Effects on rate of dialysis and on the retention of nutrients by the chick. Cereal Chemistry, 65: 298–302.
GONZÁLEZ-PASTOR, J.E., HOBBS, E.C. & LOSICK, R. (2003) Cannibalism by sporulating bacteria. Science, 301: 510–513. doi:10.1126/science.1086462 GRAMMER, J.C., MCGINNIS, J. & PUBOLS, M.H. (1982) The effects of a pectic enzyme on the growth-depressing and rachitogenic properties of rye for chicks. Poultry Science, 61: 1891– 1896. doi:10.3382/ps.0611891
HENDRICKS, C.W., DOYLE, J.D. & HUGLEY, B. (1995) A new solid medium for enumerating cellulose-utilizing bacteria in soil. Applied and Environmental Microbiology, 61: 2016–2019.
HIGGINS, D. & DWORKIN, J. (2012) Recent progress in Bacillus subtilis sporulation. FEMS Microbiology Reviews, 36: 131–148. doi:10.1111/j.1574-6976.2011.00310.x
HONG, H.A., DUC, L.H. & CUTTING, S.M. (2005) The use of bacterial spore formers as probiotics. FEMS Microbiology Reviews, 29: 813–835. doi:10.1016/j.femsre.2004.12.001
HONG, H.A., KHANEJA, R., TAM, N.M., CAZZATO, A., TAN, S., URDACI, M., BRISSON, A., GASBARRINI, A., BARNES, I. & CUTTING, S.M. (2009) Bacillus subtilis isolated from the human gastrointestinal tract. Research in Microbiology, 160: 134–143. doi:10.1016/j.resmic.2008.11.002
ILAN, Y. (2012) Leaky gut and the liver: a role for bacterial translocation in nonalcoholic steatohepatitis. World Journal of Gastroenterology, 18: 2609–2618. doi:10.3748/wjg.v18. i21.2609
JADAMUS, A., VAHJEN, H. & SIMON, O. (2001) Growth behaviour of a spore forming probiotic strain in the gastrointestinal tract of broiler chicken and piglets. Archives of Animal Nutrition, 54: 1–17.
KIARIE, E., ROMERO, L.F. & NYACHOTI, C.M. (2013) The role of added feed enzymes in promoting gut health in swine and poultry. Nutrition Research Reviews, 26: 71–88. doi:10.1017/ S0954422413000048
KUNST, F., OGASAWARA, N., MOSZER, I., ALBERTINI, A., ALLONI, G., AZEVEDO, V., BERTERO, M., BESSIERES, P., BOLOTIN, A., BORCHERT, S., & OTHERS. (1997) The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature, 390: 249–256.
LANGHOUT, D., SCHUTTE, J., GEERSE, C., KIES, A., DE JONG, J. & VERSTEGEN, M. (1997) Effects on chick performance and nutrient digestibility of an endo-xylanase added to a wheat-and rye-based diet in relation to fat source. British Poultry Science, 38: 557–563. doi:10.1080/ 00071669708418036
LATORRE, J.D., HERNANDEZ-VELASCO, X., KALLAPURA, G., MENCONI, A., PUMFORD, N.R., MORGAN, M.J., LAYTON, S.L., BIELKE, L.R., HARGIS, B.M. & TÉLLEZ, G. (2014a) Evaluation of germination, distribution, and persistence of Bacillus subtilis spores through the gastrointestinal tract of chickens. Poultry Science, 93: 1793–1800. doi:10.3382/ps.2013-03809
LATORRE, J.D., HERNANDEZ-VELASCO, X., KOGUT, M.H., VICENTE, J.L., WOLFENDEN, R., WOLFENDEN, A., HARGIS, B.M., KUTTAPPAN, V.A. & TELLEZ, G. (2014b) Role of a Bacillus subtilis direct-fed microbial on digesta viscosity, bacterial translocation, and bone mineralization in turkey poults fed with a rye-based diet. Frontiers in Veterinary Science, 1: 26. doi:10.3389/fvets.2014.00026
LATORRE, J.D., HERNANDEZ-VELAZCO, X., KUTTAPPAN, V.A., WOLFENDEN, R., VICENTE, J.L., WOLFENDEN, A., BIELKE, L., PRANDO, O., MORALES, E., HARGIS, B.M. & TELLEZ, G. (2015) Selection of Bacillus spp. for cellulase and xylanase production as direct-fed microbials to reduce digesta viscosity and Clostridium perfringens proliferation using an in vitro digestive model with different poultry diets. Frontiers in Veterinary Science, 2: 25. doi:10.3389/ fvets.2015.00025
LÁZARO, R., LATORRE, M., MEDEL, P., GRACIA, M. & MATEOS, G. (2004) Feeding regimen and enzyme supplementation to rye-based diets for broilers. Poultry Science, 83: 152–160. doi:10.1093/ps/83.2.152
LEE, K.W. (2014) Feed passage rate in broiler chickens fed on rye-based diet supplemented with essential oil components. International Journal of Poultry Science, 13: 156–159. doi:10.3923/ijps.2014.156.159
LESER, T.D., KNARREBORG, A. & WORM, J. (2008) Germination and outgrowth of Bacillus subtilis and Bacillus licheniformis spores in the gastrointestinal tract of pigs. Journal of Applied Microbiology, 104: 1025–1033. doi:10.1111/j.1365- 2672.2007.03633.x
LÓPEZ, D., VLAMAKIS, H., LOSICK, R. & KOLTER, R. (2009) Cannibalism enhances biofilm development in Bacillus subtilis. Molecular Microbiology, 74: 609–618. doi:10.1111/j.1365- 2958.2009.06882.x
MACAULIFFE, T. & MCGINNIS, J. (1971) Effect of antibiotic supplements to diets containing rye on chick growth. Poultry Science, 50: 1130–1134. doi:10.3382/ps.0501130
MACAULIFFE, T., PIETRASZEK, A. & MCGINNIS, J. (1976) Variable rachitogenic effects of grain and alleviation by extraction or supplementation with Vitamin D, fat and antibiotics. Poultry Science, 55: 2142–2147. doi:10.3382/ ps.0552142
MENCONI, A., MORGAN, M.J., PUMFORD, N.R., HARGIS, B.M. & TELLEZ, G. (2013) Physiological properties and Salmonella growth inhibition of probiotic Bacillus strains isolated from environmental and poultry sources. International Journal of Bacteriology, 2013: 1–8.
MONISHA, R., UMA, M.V. & KRISHNA MURTHY, V. (2009) Partial purification and characterization of Bacillus pumilus xylanase from soil source. Kathmandu University Journal of Science, 5: 137–148.
NATIONAL RESEARCH COUNCIL (1994) Nutrient requirements of poultry. 9th rev. edn. (Washington, DC, National Academy Press). SAS INSTITUTE (2002) SAS user guide. Version 9.1. (Cary, NC, SAS Institute Inc.).
SEKI, E. & SCHNABL, B. (2012) Role of innate immunity and the microbiota in liver fibrosis: crosstalk between the liver and gut. The Journal of Physiology, 590: 447–458. doi:10.1113/ tjp.2012.590.issue-3
SEN, S., INGALE, S., KIM, Y.W., KIM, J.S., KIM, K.H., LOHAKARE, J.D., KIM, E.K., KIM, H.S., RYU, M.H., KWON, I.K. & CHAE, J.B. (2012) Effect of supplementation of Bacillus subtilis LS 1-2 to broiler diets on growth performance, nutrient retention, caecal microbiology and small intestinal morphology. Research in Veterinary Science, 93: 264–268. doi:10.1016/j.rvsc.2011.05.021
SHIVARAMAIAH, S., PUMFORD, N., MORGAN, M., WOLFENDEN, R., WOLFENDEN, A., TORRES-RODRIGUEZ, A., HARGIS, B. & TÉLLEZ, G. (2011) Evaluation of Bacillus species as potential candidates for direct-fed microbials in commercial poultry. Poultry Science, 90: 1574–1580. doi:10.3382/ ps.2010-00745
SILVA, S.S.P. & SMITHARD, R.R. (2002) Effect of enzyme supplementation of a rye-based diet on xylanase activity in the small intestine of broilers, on intestinal crypt cell proliferation and on nutrient digestibility and growth performance of the birds. British Poultry Science, 43: 274–282. doi:10.1080/ 00071660120121508
TELLEZ, G., LATORRE, J.D., KUTTAPPAN, V.A., KOGUT, M.H., WOLFENDEN, A., HERNANDEZ-VELASCO, X., HARGIS, B.M., BOTTJE, W.G., BIELKE, L.R. & FAULKNER, O.B. (2014) Utilization of rye as energy source affects bacterial translocation, intestinal viscosity, microbiota composition, and bone mineralization in broiler chickens. Frontiers in Genetics, 5: 339. doi:10.3389/fgene.2014.00339
TELLEZ, G., PIXLEY, C., WOLFENDEN, R.E., LAYTON, S.L. & HARGIS, B.M. (2012) Probiotics/direct fed microbials for Salmonella control in poultry. Food Research International, 45: 628–633. doi:10.1016/j.foodres.2011.03.047
TELLEZ, G., RODRÍGUEZ-FRAGOSO, L., KUTTAPPAN, V.A., KALLAPURA, G., HERNANDEZ-VELASCO, X., MENCONI, A., LATORRE, J.D., WOLFENDEN, A.D., HARGIS, B.M. & REYES-ESPARZA, J. (2013) Probiotics for human and poultry use in the control of gastrointestinal disease: A Review of real-world experiences. Alternative and Integrative Medicine, 2: 118.
WIDEMAN, R.F. & PRISBY, R.D. (2013) Bone circulatory disturbances in the development of spontaneous bacterial chondronecrosis with osteomyelitis: a translational model for the pathogenesis of femoral head necrosis. Frontiers in Endocrinology, 3: 1–14.
WOLFENDEN, R., PUMFORD, N., MORGAN, M., SHIVARAMAIAH, S., WOLFENDEN, A., PIXLEY, C., GREEN, J., TELLEZ, G. & HARGIS, B. (2011) Evaluation of selected direct-fed microbial candidates on live performance and Salmonella reduction in commercial turkey brooding houses. Poultry Science, 90: 2627–2631. doi:10.3382/ps.2011-01360
ZHANG, B. & COON, C.N. (1997) The relationship of various tibia bone measurements in hens. Poultry Science, 76: 1698– 1701. doi:10.1093/ps/76.12.1698
ZHAO, S., DENG, L., HU, N., ZHAO, B. & LIANG, Y. (2008) Costeffective production of Bacillus licheniformis using simple netting bag solid bioreactor. World Journal of Microbiology and Biotechnology, 24: 2859–2863. doi:10.1007/s11274-008- 9820-5


















.jpg&w=3840&q=75)