Lipid Regulation of Gene Expression in Ruminants
Published: July 25, 2014
By: Juan Loor and Afshin Hosseini (University of Illinois)
Introduction
The advent of molecular tools and species-specific reagents over the last two decades has substantially advanced the knowledge of the molecular responses to nutrients in tissues of ruminant animals. Several comprehensive reviews on the topic of lipid regulation of gene expression in ruminants exist [1-3]. The emphasis of the current work was to provide a concise review of the most-recent work evaluating the regulation of gene expression in ruminant tissues by dietary lipids. The review is focused on key tissues involved in lipid metabolism (liver, adipose, and mammary), emphasizing data generated in vivo and in vitro.
Liver
In Vivo
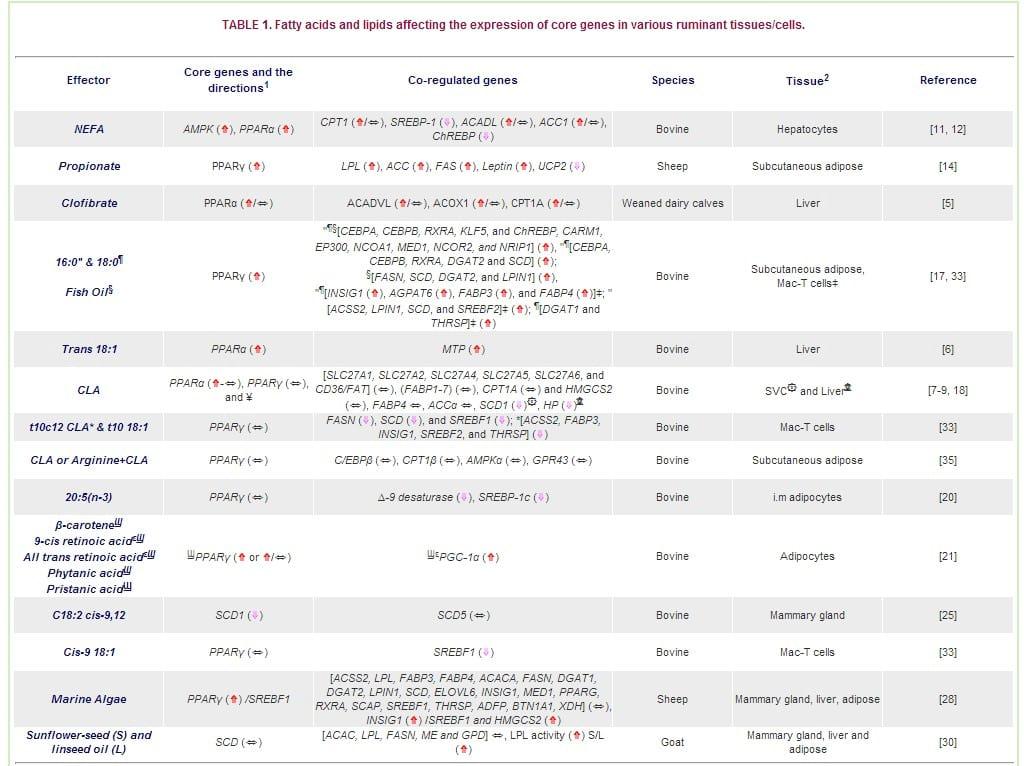
Liver is a pivotal organ regulating the metabolism of lipid, carbohydrate and protein. Negative energy balance during the peripartal period, i.e. 3 weeks prior through 3 weeks after parturition or nutrient restriction decrease the concentration of blood glucose and increase concentration of circulating nonesteri?ed fatty acids (NEFA) and β-hydroxybutyrate (BOHB) in the blood. Accumulation of triacylglycerol in liver often follows. Hepatic mechanisms such as peroxisomal oxidation of long chain fatty acids during the transition period are crucial for influencing lipid metabolism pathways; results of several recent studies indicated that peroxisomal oxidation is induced by long-chain fatty acids through greater transcription of Acyl-Coenzyme A Oxidase 1 (ACOX1), Long chain acyl-CoA dehydrogenase (ACADVL), Carnitine palmitoyltransferase 1A (CPT1A) genes, which appear to be regulated partly by peroxisome proliferator-activated receptor-alpha (PPAR-α) [4, 5]. The PPAR-α is the liver-specific isotype of the nuclear receptor PPAR; and is a key regulator of transcription of many genes involved in lipid transport and oxidation [3].
Saturated and unsaturated long-chain fatty acids induced the transactivation of PPAR-α in humans. In dairy cows, dietary trans octadecenoic acids upregulated the expression of hepatic PPAR-α during the first month of lactation [6] (Table 1). It was speculated that the increase in mRNA of microsomal triglyceride transfer protein (MTP), which is a heterodimeric lipid transfer protein regulated in non-ruminants partly by PPAR-α, plays a crucial role in the assembly and secretion of very low density lipoprotein (VLDL) in the liver [6]; the results of that study suggested the potential for supplemental dietary trans fatty acids to reduce the onset of liver lipidosis in peripartal dairy cattle.
Saturated and unsaturated long-chain fatty acids induced the transactivation of PPAR-α in humans. In dairy cows, dietary trans octadecenoic acids upregulated the expression of hepatic PPAR-α during the first month of lactation [6] (Table 1). It was speculated that the increase in mRNA of microsomal triglyceride transfer protein (MTP), which is a heterodimeric lipid transfer protein regulated in non-ruminants partly by PPAR-α, plays a crucial role in the assembly and secretion of very low density lipoprotein (VLDL) in the liver [6]; the results of that study suggested the potential for supplemental dietary trans fatty acids to reduce the onset of liver lipidosis in peripartal dairy cattle.
1 Consequence of effector on mRNA expression of the gene (⇑ induction; ⇓ inhibition; ⇑/⇔ tendency; ⇔ no change).
2?Mac-T = Bovine Mammary Epithelial Cell Line
¥The PPARs were not measured
2?Mac-T = Bovine Mammary Epithelial Cell Line
¥The PPARs were not measured
The polyunsaturated fatty acids (PUFA), in particular conjugated linoleic acid (CLA) have been used to manipulate gene expression albeit with different effects on mRNA expression of PPAR-α and PPAR-γ (Table 1). In one study with dairy cows, results did not reveal significant effects of CLA supplementation on expression in liver tissue of PPAR-α, PPAR-γ, sterol regulatory element-binding protein-1 (SREBP1) or tumor necrosis factor-alpha (TNF-α) [7]. It is well-known in non-ruminants that CLA decreases concentration of the pro-inflammatory cytokines. In addition, the PUFA evoke metabolic changes influencing the transcription of genes encoding proteins for lipid synthesis or oxidation. The data from a recent study with dairy cattle revealed that CLA supplementation in early lactation improved energy balance at a dose that effectively caused milk fat depression with no effect on hepatic lipid metabolism [8]. The results of the study indicated that the genes encoding hepatic fatty acid uptake (Solute carrier family 27: SLC27A1,2,4,5,6, Cluster of Differentiation 36: CD36/FAT and Fatty acid binding proteins: FABP1-7), hepatic mitochondrial (CPT1A) and peroxisomal β-oxidation (3-hydroxy-3-methylglutaryl-CoA synthase 2: HMGCS2) enzymes were not significantly affected in the CLA-supplemented group compared with control cows [8].
A summary of non-ruminant data compiled by Reynolds and Roche (2010) revealed that CLA could have anti-inflammatory effects through inhibition of NF-kB signaling proteins, activation of PPAR-γ and/or reduction of cytokine levels. However, the results of a recent study in dairy cattle do not support the anti-inflammatory effect of CLA on serum Haptoglobin (Hp) and liver Hp mRNA [9]. The anti-inflammatory effect of CLA on Hp was observed in omental and subcutaneous fat at 42 d and 105 d post-partum. Thus, these results highlight the tissue-specific effect of CLA in dairy cattle [9] (Table 1).
In vitro
Historically it has been very challenging to culture adult bovine hepatocytes which means few published studies are available and most did not involve measurement of gene expression [10]. However, a few recent papers have uncovered the effect of NEFA in combination with long chain fatty acids on gene expression in bovine hepatocytes [11, 12]. Other recent data in bovine kidney cell lines revealed that palmitic acid compared with PUFA had a more pronounced effect on PPAR-α-regulated genes [13]. Increasing the NEFA concentration in culture medium upregulated CPT1A in bovine hepatocytes indicating that one mechanism for the control of mitochondrial β-oxidation is at the transcriptional level [11, 12]. The results of that study also revealed that very high NEFA concentrations might inhibit the translocation of long-chain fatty acids into mitochondria [12]. The NEFA might activate the AMP-Activated Protein Kinase (AMPK) signaling pathway in bovine hepatocytes to trigger lipid metabolism-related enzymes, e.g. up-regulation of ACSL and CPT1, and down-regulation of ACC1 (inhibition of fatty acid synthesis) as a global mechanism favoring fatty acid oxidation [11]. Therefore, long-chain fatty acids in a dose-dependent manner could modify lipid signal transduction pathways.
Adipose Tissue
In vivo
The infusion of the short-chain fatty acid propionate increased the mRNA expression of the PPAR-γ and its target genes (lipoprotein lipase (LPL), acetyl CoA carboxylase (ACC), fatty acid synthase (FASN) in subcutaneous adipose tissue of sheep [14] (Table 1). The results of the study suggested that the short-term infusion of short-chain fatty acids stimulated lipogenesis in adipose tissue [14]. It has been known that not only the short-chain fatty acids are key energy sources in ruminants but also they interact with other biological pathways such as insulin signaling [15]. The short-chain fatty acid propionate increased the blood concentration of insulin in dairy cattle in vivo.
Conjugated linoleic acids markedly increased glucose-stimulated insulin secretion in mice partly through the free fatty acid receptor; however, the effect of CLA on insulin sensitivity in humans is controversial. It has been reported that the CLA isomer cis-9,trans-11 increases insulin sensitivity and inhibits proinflammatory cytokines, while the trans-10,cis-12 isomer triggers the inflammatory response and promotes insulin resistance in primary cultures of human adipocytes. In non-ruminants, increased visceral adipose tissue is associated with impaired liver metabolism, increased insulin resistance and incidence of metabolic disease. In contrast with non-ruminant species, body fat mass remains largely unchanged by CLA treatment in dairy cows, except for the retroperitoneal depot [16].
Diets supplemented with saturated long-chain fatty acids (mostly 16:0 and 18:0) or fish oil during the transition period altered PPAR-γ co-regulator and target gene expression in subcutaneous adipose tissue of dairy cattle [17] (Table 1). The results of that study uncovered upregulation of the adipogenic/lipogenic transcription regulators [CEBPA, CEBPB, RXRA, KLF5, and MLXIPL (formerly ChREBP)] and co-regulators (CARM1, EP300, NCOA1, MED1, NCOR2, and NRIP1) by fish oil and saturated long-chain fatty acids during the prepartal period. In addition, fish oil activated lipogenesis/triacylglycerol synthesis (FASN, SCD, DGAT2, and LPIN1) enzymes [17]. Supplementing saturated long-chain fatty acids compared with fish oil led to greater expression of CEBPA, CEBPB, RXRA, several PPAR-co-activators, and DGAT2 and SCD by sustaining a pro-adipogenic/pro-lipogenic state [17].
In vitro
Trans-10,cis-12 CLA inhibited the differentiation of bovine stromal-vascular cells into mature adipocytes, reducing radiolabeled acetate incorporation into lipids, stearoyl-CoA desaturase-1 (SCD) and ACCα protein abundance [18]. The long-term incubation of the stromal-vascular cells with CLA had no effect on the mRNA expression of FABP4, PPAR-γ, or ACCα, but there was a reduction in the mRNA expression of SCD1 compared with cells cultured with linoleic acid and control [18]. The mRNA of SCD was down-regulated by the mRNA of PPAR-γ [18]. The evaluation of several studies underscored the inhibitory effect of CLA on differentiation of adipocytes and preadipocyes in meat animals [19]. The results of an in vitro study with bovine intramuscular adipocyte cell cultures treated with eicosapentaenoic acid uncovered that the increasing dosage of this fatty acid decreased the mRNA expression of ?9 desaturase and SREBP-1c [20]. It could be possible that eicosapentaenoic acid triggers de novo lipogenesis via SREBP-1c in bovine intramuscular adipocytes.
Long-chain fatty acids differentially affect PPAR-γ and its co-activators in preadipocytes obtained from bovine white adipose tissue [21]. The results of a study showed that phytanic and pristanic acids, vitamin A (all-trans- and 9-cis-retinoic acid), and carotenoids (β-carotene and lutein) influence the expression of PPAR-γ and its coactivator PGC-1α during differentiation in bovine white adipose tissue; however, the 9-cis-retinoic acid was the most potent inducer of PPAR-γ expression [21]. Furthermore, phytanic acid had a greater effect on mRNA of PPAR-γ compared with pristanic acid [21] (Table 1). Thus, the results of this study uncovered a potential difference in the potency of different fatty acids on PPAR-γ activation and adipogenesis.
Mammary Tissue
In vivo
Increasing exogenous CLA (t10,c12-CLA) reduced milk fat synthesis [23]; the reduction was less pronounced for exogenous c9,t11-CLA [24]. The results of the study underscored that high doses of t10,c12-CLA reduced de novo synthesized fatty acids and desaturation of 18:0 via ?9-desaturase (SCD) [23]. Although the dietary supplementation of soybean oil and linseed oil increased the milk fat proportion of C18:2 cis-9,12 and C18:3 cis-9,12,15, respectively, the mRNA expression of the SCD1 gene in mammary gland decreased with the soybean oil supplement compared with rapeseed and linseed oil. In contrast, the mRNA expression of SCD5 remained unchanged [25]. The results underscored that the activity of ?9-desaturase (evaluated via desaturation ratios using milk fatty acid data) is correlated with milk fatty acids within the mammary gland. The negative effect of dietary C18:2 cis-9,12 on SCD1 expression has been well-described in non-ruminants.
Further microarray-based studies with rumen-protected CLA supplementation indicated that the CLA marginally affects fatty acid distribution of body tissues with no effect on gene expression of core lipid metabolism triggering genes such as PPARs, ACADVL, CPT1A, and FABPs in the mammary gland of heifers during early lactation [27]. It is possible that in this study poor protection of ruminal CLA led to elevated concentrations of t-18:1 fatty acids without affecting the lipid metabolism pathways in main organs such as mammary gland and adipose tissue. Moreover, it appears that based on an average dry matter intake, the dose of the supplemented CLA isomers was lower compared with similar types of studies with rodent or pig models.
To date several studies have been performed in small ruminants that demonstrate the effect of long-chain fatty acid supplementation on milk fat depression without marked changes in mRNA of lipogenic targets. In one such study, dairy ewes were fed a linoleic acid-rich diet with added marine algae, resulting in reduced milk fat content and alterations in milk fatty acid profile through increased abundance of PPAR-γ and INSIG1 in mammary tissue compared with SREBF1 [28]. In accordance with previous reports, the results of the study indicated no change in expression of genes related to fatty acid uptake and activation, transport, de novo fatty acid synthesis, esterification, desaturation, elongation, transcriptional regulation and lipid droplet formation [28]. Notably, similar types of lipids caused an expected increase in mRNA abundance of PPAR-γ targets (i.e. LPIN1 and SREBF1 along with ACSS2, ACACA, FASN, and LPL) during the first 7 d of feeding, but by 21 d of feeding the mRNA expression returned to basal levels [29]. Thus, differences between bovine and caprine/ovine species in the molecular response to dietary fat seem to exist.
It seems likely, as in dairy cattle, that the PPAR-γ is the core regulator of body lipid metabolism in dairy ewes. Further studies with oil supplementation in goats uncovered that plant oils suppress milk fat synthesis but have no effect on mammary SCD, acetyl-CoA carboxylase (ACAC), LPL, FASN, malic enzyme (ME) and glycerol-3-phosphate dehydrogenase (GPD) mRNA or activity [30]. However, the activity of LPL was increased in mammary gland with increasing sunflower-seed oil relative to linseed oil, while no degree of change was observed for the mRNA of GPD, ME, GPD activity, or mRNA abundance and/or activity of LPL, ACAC, FASN, and SCD in liver or adipose tissue [30]. The evaluation of these data underscore that SCD targeted by PPAR-γ is the core trigger of fat synthesis in mammary gland of ruminants fed with dietary lipid supplements.
In vitro
The results of recent studies evidenced that PPAR-γ along with its lipogenic targets in mammary gland are activated during lactation in dairy cattle [31, 32]. The treatment of Mac-T cells with several long-chain fatty acids for 12 h differentially affected the genes regulating lipid metabolism in vitro [33]. It has been reported that CLA increases the activity of PPAR targets in bovine Mac-T cells. In similar fashion, the coordinated upregulation of lipogenic gene networks in response to PPAR-γ ligands and saturated long-chain fatty acids offer support for PPAR-γ activation in regulating bovine milk fat synthesis [33].
It should be noted that recent findings suggest that Mac-T cells might be an adequate model to study milk protein synthesis regulation but not sensitive compared with mammary tissue for studies of milk fat synthesis regulation [34]. The Mac-T cells are responsive to long-chain fatty acids. The responses of Mac-T cells to 16:0 and 18:0 treatments led to greater expression of intracellular fatty acid transport, lipid droplet formation (16:0) and esterification (18:0), while the treatment of the cells with t10-18:1 and t10c12 CLA reduced desaturation, transcriptional regulation and de novo fatty acid synthesis after 12 h of incubation. Thus, it might be speculated that most of the target genes of PPAR-γ were co-regulated by fatty acid treatments.
Conclusions
Dietary long-chain fatty acid supplementation in ruminants potentially may affect the direction and dimension of changes in lipid metabolism gene networks in key physiological organs such as liver, adipose tissue and mammary gland. Through effects at the gene level, dietary fatty acids can regulate fatty acid uptake and activation, intracellular transport, de novo fatty acid synthesis, esterification, desaturation, and lipid droplet formation. Some of the data available highlight differences among ruminant species in terms of the molecular response to dietary fatty acids. Emerging data underscore the role of PPARs as a core regulator of metabolic genes in livestock; therefore, the data suggest that dietary fatty acids could act as natural ligands for PPARs. If this relationship can be fine-tuned, processes such as milk fat synthesis, hepatic fatty acid oxidation, and adipocyte differentiation might be regulated partly via lipid nutrition.
References
Bernard, L., Leroux, C. and Chilliard, Y. Expression and nutritional regulation of lipogenic genes in the ruminant lactating mammary gland. Adv. Exp.Med. Biol., 606, 67-108 (2008). (DOI: 10.1007/978-0-387-74087-4_2).
Bauman, D.E., Harvatine, K.J. and Lock, A.L. Nutrigenomics, rumen-derived bioactive fatty acids, and the regulation of milk fat synthesis. Annu. Rev. Nutr., 31, 299-319 (2011) (DOI: 10.1146/annurev.nutr.012809.104648).
Bionaz, M., Chen, S., Khan, M.J. and Loor, J.J. Functional role of PPARs in ruminants: potential targets for fine-tuning metabolism during growth and lactation. PPAR Research 2013, 684159 (2013) (DOI: 10.1155/2013/684159).
Grum, D.E., Drackley, J.K., Younker, R.S., LaCount, D.W. and Veenhuizen, J.J. Nutrition during the dry period and hepatic lipid metabolism of periparturient dairy cows. J. Dairy Sci., 79, 1850-1864 (1996) (DOI: 10.3168/jds.S0022-0302(96)76553-0).
Litherland, N.B., Bionaz, M., Wallace, R.L., Loor, J.J. and Drackley, J.K. Effects of the peroxisome proliferator-activated receptor-α agonists clofibrate and fish oil on hepatic fatty acid metabolism in weaned dairy calves. J. Dairy Sci., 93, 2404-2418 (2010) (DOI: 10.3168/jds.2009-2716).
Selberg, K.T., Staples, C.R., Luchini, N.D. and Badinga, L. Dietary trans octadecenoic acids upregulate the liver gene encoding peroxisome proliferator-activated receptor-alpha in transition dairy cows. J. Dairy Res., 72, 107-114 (2005) (DOI: 10.1017/S0022029904000573).
Sigl, T., Schlamberger, G., Kienberger, H., Wiedemann, S., Meyer, H.H. and Kaske, M. Rumen-protected conjugated linoleic acid supplementation to dairy cows in late pregnancy and early lactation: effects on milk composition, milk yield, blood metabolites and gene expression in liver. Acta Vet. Scand., 52, 16 (2010) (DOI: 10.1186/1751-0147-52-16).
Schlegel, G., Ringseis, R., Windisch, W., Schwarz, F.J. and Eder, K. Effects of a rumen-protected mixture of conjugated linoleic acids on hepatic expression of genes involved in lipid metabolism in dairy cows. J. Dairy Sci., 95, 3905-3918 (2012) (DOI: 10.3168/jds.2011-4835).
Saremi, B., Al-Dawood, A., Winand, S., Muller, U., Pappritz, J., von Soosten, D., Rehage, J., Danicke, S., Haussler, S., Mielenz, M. et al. Bovine haptoglobin as an adipokine: serum concentrations and tissue expression in dairy cows receiving a conjugated linoleic acids supplement throughout lactation. Vet. Immunol. Immunopathol., 146, 201-211 (2012) (DOI: 10.1016/j.vetimm.2012.03.011).
Mashek, D.G. and Grummer, R.R. Effects of long chain fatty acids on lipid and glucose metabolism in monolayer cultures of bovine hepatocytes. J. Dairy Sci., 86, 2390-2396 (2003) (DOI: 10.3168/jds.S0022-0302(03)73833-8).
Li, X., Li, X., Chen, H., Lei, L., Liu, J., Guan, Y., Liu, Z., Zhang, L., Yang, W., Zhao, C. et al. Non-esterified fatty acids activate the AMP-activated protein kinase signaling pathway to regulate lipid metabolism in bovine hepatocytes. Cell Biochem. Biophys., 67, 1157-1169 (2013) (DOI: 10.1007/s12013-013-9629-1).
Li, P., Liu, Y., Zhang, Y., Long, M., Guo, Y., Wang, Z., Li, X., Zhang, C., Li, X., He, J. et al. Effect of non-esterified fatty acids on fatty acid metabolism-related genes in calf hepatocytes cultured in vitro. Cell. Physiol. Biochem: Int. J. Exp. Cell. Physiol., Biochem. Pharmacol., 32, 1509-1516 (2013) (DOI: 10.1159/000356588).
Bionaz, M., Thering, B.J. and Loor, J.J. Fine metabolic regulation in ruminants via nutrient-gene interactions: saturated long-chain fatty acids increase expression of genes involved in lipid metabolism and immune response partly through PPAR-α activation. Brit. J. Nutr., 107, 179-191 (2012) (DOI: 10.1017/S0007114511002777).
Lee, S.H. and Hossner, K.L. Coordinate regulation of ovine adipose tissue gene expression by propionate. J. Anim. Sci., 80, 2840-2849 (2002).
Hosseini, A., Behrendt, C., Regenhard, P., Sauerwein, H. and Mielenz, M. Differential effects of propionate or β-hydroxybutyrate on genes related to energy balance and insulin sensitivity in bovine white adipose tissue explants from a subcutaneous and a visceral depot. J. Anim. Physiol. Anim. Nutr., 96, 570-580 (2011) (DOI: 10.1111/j.1439-0396.2011.01180.x).
von Soosten, D., Meyer, U., Weber, E.M., Rehage, J., Flachowsky, G.. and Danicke, S. Effect of trans-10, cis-12 conjugated linoleic acid on performance, adipose depot weights, and liver weight in early-lactation dairy cows. J. Dairy Sci., 94, 2859-2870 (2011) (DOI: 10.3168/jds.2010-3851).
Schmitt, E., Ballou, M.A., Correa, M.N., DePeters, E.J., Drackley, J.K. and Loor, J.J. Dietary lipid during the transition period to manipulate subcutaneous adipose tissue peroxisome proliferator-activated receptor-gamma co-regulator and target gene expression. J. Dairy Sci., 94, 5913-5925 (2011) (DOI: 10.3168/jds.2011-4230).
Lengi, A.J. and Corl, B.A. Factors influencing the differentiation of bovine preadipocytes in vitro. J. Anim. Sci., 88, 1999-2008 (2010) (DOI: 10.2527/jas.2009-2439).
Hausman, G.J., Dodson, M.V., Ajuwon, K., Azain, M., Barnes, K.M., Guan, L.L., Jiang, Z., Poulos, S.P., Sainz, R.D., Smith, S. et al. Board-invited review: the biology and regulation of preadipocytes and adipocytes in meat animals. J. Anim. Sci., 87, 1218-1246 (2009) (DOI: 10.2527/jas.2008-1427).
Waters, S.M., Kenny, D.A., Killeen, A.P., Spellman, S.A., Fitzgerald, A., Hennessy, A.A. and Hynes, A.C. Effect of level of eicosapentaenoic acid on the transcriptional regulation of Δ-9 desaturase using a novel in vitro bovine intramuscular adipocyte cell culture model. Animal, 3, 718-727 (2009) (DOI: 10.1017/S1751731109004054).
Garcia-Rojas, P., Antaramian, A., Gonzalez-Davalos, L., Villarroya, F., Shimada, A., Varela-Echavarria, A. and Mora, O. Induction of peroxisomal proliferator-activated receptor gamma and peroxisomal proliferator-activated receptor γ coactivator 1 by unsaturated fatty acids, retinoic acid, and carotenoids in preadipocytes obtained from bovine white adipose tissue. J. Anim. Sci., 88, 1801-1808 (2010) (DOI: 10.2527/jas.2009-2579).
Mach, N., van Baal, J., Kruijt, L., Jacobs, A. and Smits, M. Dietary unsaturated fatty acids affect the mammary gland integrity and health in lactating dairy cows. BMC Proc., 5 Suppl. 4, S35 (2011) (DOI: 10.1186/1753-6561-5-S4-S35).
Baumgard, L.H., Sangster, J.K. and Bauman, D.E. Milk fat synthesis in dairy cows is progressively reduced by increasing supplemental amounts of trans-10, cis-12 conjugated linoleic acid (CLA). J. Nutr., 131, 1764-1769 (2001).
Perfield, J.W., Lock, A.L., Griinari, J.M., Saebo, A., Delmonte, P., Dwyer, D.A. and Bauman, D.E. Trans-9, cis-11 conjugated linoleic acid reduces milk fat synthesis in lactating dairy cows. J. Dairy Sci., 90, 2211-2218 (2007) (DOI: 10.3168/jds.2006-745).
Jacobs, A.A.A., van Baal, J., Smits, M.A., Taweel, H.Z., Hendriks, W.H., van Vuuren, A.M. and Dijkstra, J. Effects of feeding rapeseed oil, soybean oil, or linseed oil on stearoyl-CoA desaturase expression in the mammary gland of dairy cows. J. Dairy Sci., 94, 874-887 (2011) (DOI: 10.3168/jds.2010-3511).
Jacobs, A.A.A., Dijkstra, J., ndriks, W.H., van Baal, J. and van Vuuren, A.M. Comparison between stearoyl-CoA desaturase expression in milk somatic cells and in mammary tissue of lactating dairy cows. J. Anim. Physiol. Anim. Nutr., 97, 353-362 (2013) ( 10.1111/j.1439-0396.2012.01278.x).
Kramer, R., Wolf, S., Petri, T., von Soosten, D., Danicke, S., Weber, E.M., Zimmer, R., Rehage, J. and Jahreis, G. A commonly used rumen-protected conjugated linoleic acid supplement marginally affects fatty acid distribution of body tissues and gene expression of mammary gland in heifers during early lactation. Lipids Health Dis., 12, 96 (2013) (DOI: 10.1186/1476-511X-12-96).
Bichi, E., Frutos, P., Toral, P.G., Keisler, D., Hervás, G. and Loor, J.J. Dietary marine algae and its influence on tissue gene network expression during milk fat depression in dairy ewes. Anim. Feed Sci. Technol., 186, 36-44 (2013) (DOI: 10.1016/j.anifeedsci.2013.09.010).
Invernizzi, G., Thering, B.J., McGuire, M.A., Savoini, G. and Loor, J.J. Sustained upregulation of stearoyl-CoA desaturase in bovine mammary tissue with contrasting changes in milk fat synthesis and lipogenic gene networks caused by lipid supplements. Funct. Integr. Genom., 10, 561-575 (2010) (DOI: 10.1007/s10142-010-0179-y).
Bernard, L., Bonnet, M., Leroux, C., Shingfield, K.J. and Chilliard, Y. Effect of sunflower-seed oil and linseed oil on tissue lipid metabolism, gene expression, and milk fatty acid secretion in Alpine goats fed maize silage-based diets. J. Dairy Sci., 92, 6083-6094 (2009) (DOI: 10.3168/jds.2009-2048).
Gao, Y., Lin, X., Shi, K., Yan, Z. and Wang, Z. Bovine mammary gene expression profiling during the onset of lactation. PloS One, 8, e70393 (2013) (DOI: 10.1371/journal.pone.0070393).
Bionaz, M. and Loor, J.J. Gene networks driving bovine milk fat synthesis during the lactation cycle. BMC Genomics, 9, 366 (2008) (DOI: 10.1186/1471-2164-9-366).
Kadegowda, A.K., Bionaz, M, Piperova, L.S., Erdman, R.A. and Loor, J.J. Peroxisome proliferator-activated receptor-γ activation and long-chain fatty acids alter lipogenic gene networks in bovine mammary epithelial cells to various extents. J. Dairy Sci., 92, 4276-4289 (2009) (DOI: 10.3168/jds.2008-1932).
Hosseini, A., Sharma, R., Bionaz, M. and Loor, J.J. Transcriptomics comparisons of Mac-T cells versus mammary tissue during late pregnancy and peak lactation. Adv. Dairy Res., 1, 103 (2013) (DOI: 10.4172/2329-888X.1000103).
Choi, S.H., Wickersham, T.A., Wu, G., Gilmore, L.A., Edwards, H.D., Park, S.K., Kim, K.H. and Smith, S.B. Abomasal infusion of arginine stimulates SCD and C/EBPβ gene expression, and decreases CPT1β gene expression in bovine adipose tissue independent of conjugated linoleic acid. Amino Acids, 46, 353-366 (2014) (DOI: 10.1007/s00726-013-1622-x).
Bauman, D.E., Harvatine, K.J. and Lock, A.L. Nutrigenomics, rumen-derived bioactive fatty acids, and the regulation of milk fat synthesis. Annu. Rev. Nutr., 31, 299-319 (2011) (DOI: 10.1146/annurev.nutr.012809.104648).
Bionaz, M., Chen, S., Khan, M.J. and Loor, J.J. Functional role of PPARs in ruminants: potential targets for fine-tuning metabolism during growth and lactation. PPAR Research 2013, 684159 (2013) (DOI: 10.1155/2013/684159).
Grum, D.E., Drackley, J.K., Younker, R.S., LaCount, D.W. and Veenhuizen, J.J. Nutrition during the dry period and hepatic lipid metabolism of periparturient dairy cows. J. Dairy Sci., 79, 1850-1864 (1996) (DOI: 10.3168/jds.S0022-0302(96)76553-0).
Litherland, N.B., Bionaz, M., Wallace, R.L., Loor, J.J. and Drackley, J.K. Effects of the peroxisome proliferator-activated receptor-α agonists clofibrate and fish oil on hepatic fatty acid metabolism in weaned dairy calves. J. Dairy Sci., 93, 2404-2418 (2010) (DOI: 10.3168/jds.2009-2716).
Selberg, K.T., Staples, C.R., Luchini, N.D. and Badinga, L. Dietary trans octadecenoic acids upregulate the liver gene encoding peroxisome proliferator-activated receptor-alpha in transition dairy cows. J. Dairy Res., 72, 107-114 (2005) (DOI: 10.1017/S0022029904000573).
Sigl, T., Schlamberger, G., Kienberger, H., Wiedemann, S., Meyer, H.H. and Kaske, M. Rumen-protected conjugated linoleic acid supplementation to dairy cows in late pregnancy and early lactation: effects on milk composition, milk yield, blood metabolites and gene expression in liver. Acta Vet. Scand., 52, 16 (2010) (DOI: 10.1186/1751-0147-52-16).
Schlegel, G., Ringseis, R., Windisch, W., Schwarz, F.J. and Eder, K. Effects of a rumen-protected mixture of conjugated linoleic acids on hepatic expression of genes involved in lipid metabolism in dairy cows. J. Dairy Sci., 95, 3905-3918 (2012) (DOI: 10.3168/jds.2011-4835).
Saremi, B., Al-Dawood, A., Winand, S., Muller, U., Pappritz, J., von Soosten, D., Rehage, J., Danicke, S., Haussler, S., Mielenz, M. et al. Bovine haptoglobin as an adipokine: serum concentrations and tissue expression in dairy cows receiving a conjugated linoleic acids supplement throughout lactation. Vet. Immunol. Immunopathol., 146, 201-211 (2012) (DOI: 10.1016/j.vetimm.2012.03.011).
Mashek, D.G. and Grummer, R.R. Effects of long chain fatty acids on lipid and glucose metabolism in monolayer cultures of bovine hepatocytes. J. Dairy Sci., 86, 2390-2396 (2003) (DOI: 10.3168/jds.S0022-0302(03)73833-8).
Li, X., Li, X., Chen, H., Lei, L., Liu, J., Guan, Y., Liu, Z., Zhang, L., Yang, W., Zhao, C. et al. Non-esterified fatty acids activate the AMP-activated protein kinase signaling pathway to regulate lipid metabolism in bovine hepatocytes. Cell Biochem. Biophys., 67, 1157-1169 (2013) (DOI: 10.1007/s12013-013-9629-1).
Li, P., Liu, Y., Zhang, Y., Long, M., Guo, Y., Wang, Z., Li, X., Zhang, C., Li, X., He, J. et al. Effect of non-esterified fatty acids on fatty acid metabolism-related genes in calf hepatocytes cultured in vitro. Cell. Physiol. Biochem: Int. J. Exp. Cell. Physiol., Biochem. Pharmacol., 32, 1509-1516 (2013) (DOI: 10.1159/000356588).
Bionaz, M., Thering, B.J. and Loor, J.J. Fine metabolic regulation in ruminants via nutrient-gene interactions: saturated long-chain fatty acids increase expression of genes involved in lipid metabolism and immune response partly through PPAR-α activation. Brit. J. Nutr., 107, 179-191 (2012) (DOI: 10.1017/S0007114511002777).
Lee, S.H. and Hossner, K.L. Coordinate regulation of ovine adipose tissue gene expression by propionate. J. Anim. Sci., 80, 2840-2849 (2002).
Hosseini, A., Behrendt, C., Regenhard, P., Sauerwein, H. and Mielenz, M. Differential effects of propionate or β-hydroxybutyrate on genes related to energy balance and insulin sensitivity in bovine white adipose tissue explants from a subcutaneous and a visceral depot. J. Anim. Physiol. Anim. Nutr., 96, 570-580 (2011) (DOI: 10.1111/j.1439-0396.2011.01180.x).
von Soosten, D., Meyer, U., Weber, E.M., Rehage, J., Flachowsky, G.. and Danicke, S. Effect of trans-10, cis-12 conjugated linoleic acid on performance, adipose depot weights, and liver weight in early-lactation dairy cows. J. Dairy Sci., 94, 2859-2870 (2011) (DOI: 10.3168/jds.2010-3851).
Schmitt, E., Ballou, M.A., Correa, M.N., DePeters, E.J., Drackley, J.K. and Loor, J.J. Dietary lipid during the transition period to manipulate subcutaneous adipose tissue peroxisome proliferator-activated receptor-gamma co-regulator and target gene expression. J. Dairy Sci., 94, 5913-5925 (2011) (DOI: 10.3168/jds.2011-4230).
Lengi, A.J. and Corl, B.A. Factors influencing the differentiation of bovine preadipocytes in vitro. J. Anim. Sci., 88, 1999-2008 (2010) (DOI: 10.2527/jas.2009-2439).
Hausman, G.J., Dodson, M.V., Ajuwon, K., Azain, M., Barnes, K.M., Guan, L.L., Jiang, Z., Poulos, S.P., Sainz, R.D., Smith, S. et al. Board-invited review: the biology and regulation of preadipocytes and adipocytes in meat animals. J. Anim. Sci., 87, 1218-1246 (2009) (DOI: 10.2527/jas.2008-1427).
Waters, S.M., Kenny, D.A., Killeen, A.P., Spellman, S.A., Fitzgerald, A., Hennessy, A.A. and Hynes, A.C. Effect of level of eicosapentaenoic acid on the transcriptional regulation of Δ-9 desaturase using a novel in vitro bovine intramuscular adipocyte cell culture model. Animal, 3, 718-727 (2009) (DOI: 10.1017/S1751731109004054).
Garcia-Rojas, P., Antaramian, A., Gonzalez-Davalos, L., Villarroya, F., Shimada, A., Varela-Echavarria, A. and Mora, O. Induction of peroxisomal proliferator-activated receptor gamma and peroxisomal proliferator-activated receptor γ coactivator 1 by unsaturated fatty acids, retinoic acid, and carotenoids in preadipocytes obtained from bovine white adipose tissue. J. Anim. Sci., 88, 1801-1808 (2010) (DOI: 10.2527/jas.2009-2579).
Mach, N., van Baal, J., Kruijt, L., Jacobs, A. and Smits, M. Dietary unsaturated fatty acids affect the mammary gland integrity and health in lactating dairy cows. BMC Proc., 5 Suppl. 4, S35 (2011) (DOI: 10.1186/1753-6561-5-S4-S35).
Baumgard, L.H., Sangster, J.K. and Bauman, D.E. Milk fat synthesis in dairy cows is progressively reduced by increasing supplemental amounts of trans-10, cis-12 conjugated linoleic acid (CLA). J. Nutr., 131, 1764-1769 (2001).
Perfield, J.W., Lock, A.L., Griinari, J.M., Saebo, A., Delmonte, P., Dwyer, D.A. and Bauman, D.E. Trans-9, cis-11 conjugated linoleic acid reduces milk fat synthesis in lactating dairy cows. J. Dairy Sci., 90, 2211-2218 (2007) (DOI: 10.3168/jds.2006-745).
Jacobs, A.A.A., van Baal, J., Smits, M.A., Taweel, H.Z., Hendriks, W.H., van Vuuren, A.M. and Dijkstra, J. Effects of feeding rapeseed oil, soybean oil, or linseed oil on stearoyl-CoA desaturase expression in the mammary gland of dairy cows. J. Dairy Sci., 94, 874-887 (2011) (DOI: 10.3168/jds.2010-3511).
Jacobs, A.A.A., Dijkstra, J., ndriks, W.H., van Baal, J. and van Vuuren, A.M. Comparison between stearoyl-CoA desaturase expression in milk somatic cells and in mammary tissue of lactating dairy cows. J. Anim. Physiol. Anim. Nutr., 97, 353-362 (2013) ( 10.1111/j.1439-0396.2012.01278.x).
Kramer, R., Wolf, S., Petri, T., von Soosten, D., Danicke, S., Weber, E.M., Zimmer, R., Rehage, J. and Jahreis, G. A commonly used rumen-protected conjugated linoleic acid supplement marginally affects fatty acid distribution of body tissues and gene expression of mammary gland in heifers during early lactation. Lipids Health Dis., 12, 96 (2013) (DOI: 10.1186/1476-511X-12-96).
Bichi, E., Frutos, P., Toral, P.G., Keisler, D., Hervás, G. and Loor, J.J. Dietary marine algae and its influence on tissue gene network expression during milk fat depression in dairy ewes. Anim. Feed Sci. Technol., 186, 36-44 (2013) (DOI: 10.1016/j.anifeedsci.2013.09.010).
Invernizzi, G., Thering, B.J., McGuire, M.A., Savoini, G. and Loor, J.J. Sustained upregulation of stearoyl-CoA desaturase in bovine mammary tissue with contrasting changes in milk fat synthesis and lipogenic gene networks caused by lipid supplements. Funct. Integr. Genom., 10, 561-575 (2010) (DOI: 10.1007/s10142-010-0179-y).
Bernard, L., Bonnet, M., Leroux, C., Shingfield, K.J. and Chilliard, Y. Effect of sunflower-seed oil and linseed oil on tissue lipid metabolism, gene expression, and milk fatty acid secretion in Alpine goats fed maize silage-based diets. J. Dairy Sci., 92, 6083-6094 (2009) (DOI: 10.3168/jds.2009-2048).
Gao, Y., Lin, X., Shi, K., Yan, Z. and Wang, Z. Bovine mammary gene expression profiling during the onset of lactation. PloS One, 8, e70393 (2013) (DOI: 10.1371/journal.pone.0070393).
Bionaz, M. and Loor, J.J. Gene networks driving bovine milk fat synthesis during the lactation cycle. BMC Genomics, 9, 366 (2008) (DOI: 10.1186/1471-2164-9-366).
Kadegowda, A.K., Bionaz, M, Piperova, L.S., Erdman, R.A. and Loor, J.J. Peroxisome proliferator-activated receptor-γ activation and long-chain fatty acids alter lipogenic gene networks in bovine mammary epithelial cells to various extents. J. Dairy Sci., 92, 4276-4289 (2009) (DOI: 10.3168/jds.2008-1932).
Hosseini, A., Sharma, R., Bionaz, M. and Loor, J.J. Transcriptomics comparisons of Mac-T cells versus mammary tissue during late pregnancy and peak lactation. Adv. Dairy Res., 1, 103 (2013) (DOI: 10.4172/2329-888X.1000103).
Choi, S.H., Wickersham, T.A., Wu, G., Gilmore, L.A., Edwards, H.D., Park, S.K., Kim, K.H. and Smith, S.B. Abomasal infusion of arginine stimulates SCD and C/EBPβ gene expression, and decreases CPT1β gene expression in bovine adipose tissue independent of conjugated linoleic acid. Amino Acids, 46, 353-366 (2014) (DOI: 10.1007/s00726-013-1622-x).
Related topics:
Authors:
University of Illinois
Recommend
Comment
Share
UFAC
28 de julio de 2014
Juan,
Is my understanding right from your paper that through gene expression network PUFA can enhance lipogenesis compared with palmitic acid?
Recommend
Reply

Would you like to discuss another topic? Create a new post to engage with experts in the community.