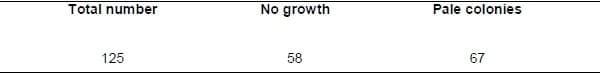1-Introduction
Avian Salmonellosis caused by Salmonella species, is one of these factors that results in economic problems concerning all stages of poultry industry from production to marketing. It causes drop of performance parameters such as egg production, fertility, hatchability and increased early chick mortality (Abd Ellatef, 1995).
Salmonellosis in chicken can be classified into three diseases conditions; Pullorum disease caused by Salmonella Pullorum; fowl Typhoid caused by Salmonella Gallinarum and paratyphoid infections due to diverse group of serovars related to food borne illness in human. Salmonella Typhimurium and more recently Salmonella Enteritidis have been the serovar most frequently isolated from case of human food poisoning in which chicken products have been implicated (Oliveira et al., 2002).
S. Enteritidis continues to be internationally important human pathogen that causes Salmonellosis (food borne zoonotic disease which may lead to food poisoning outbreaks in countries in both northern and southern hemispheres (Humphrey et al., 1996).
Traditional detection methods for Salmonella species which based on culture using selective media and characterization of suspicious colonies by biochemical and serological tests are generally time consuming (Wallance et al., 1999) while polymerase chain reaction (PCR) is considered a rapid and sensitive method for detection of S. Enteritidis (Stone et al., 1994; Abouzeed et al., 2000). Different variants could be isolated from S. Enteritidis from different places or even from the same farm. So, there is a need for typing methods depending on genotypic differences. There are different methods for genotyping of S. Enteritidis, these include serotyping, phage typing, randomly amplified polymorphic DNA (RAPD), plasmid profiling, Restriction Fragment Length Polymorphism (RFLP) and IS200, ribotyping (Chadfield et al., 2001).
Plasmid profiling is a widely used method for differentiation of salmonella isolates. Bacterial plasmid is extrachromosomal DNA elements that can replicate independently of the chromosome (Towner and Cockayne, 1993). Bacterial strains can be typed according to the plasmid content or plasmid profile of each isolate. Plasmids are not considered as a stable strain characteristic as they can be lost or undergo re-arrangement by conjunctive transfer (Liebana, 2002 and Domig et al., 2003) and therefore plasmid profiling is usually used as additional methods for typing. Some strains with the same chromosomal features may show different plasmid restriction patterns, and the same plasmid profile may be present in strains that are different at a chromosomal level (Chadfield et al., 2001; Liebana, 2002). The presence of different conformations of the same plasmid (linear or circular) may also result in altered profiles. Despites these limitations, plasmid profiling is a simple and rapid technique and can be a useful method for laboratories that are unable to perform more complex methods (Liebana, 2002).
The present study was aimed to characterize S. Enteritidis from cecal specimens of broiler chickens and confirmation by PCR. Further performing, plasmid profiling as an epidemiological typing method.
2. Materials and methods
1.1. Methods for sampling and bacteriological isolation:
A total of 125 samples were collected from liver, spleen, cecum of broiler chicks, using sterile moistened swabs and transferred into ice boxes under complete sterile measures to the lab of Microbiology Department, Faculty of veterinary medicine, Alexandria University to carry out the bacteriological isolation. The collected samples were inoculated into tetrathionate broth then incubated at 37oC for 18 hours. Then, the inoculated broth was subcultured on selective media as MacConkey agar, SS agar and Brilliant green agar. These cultures were incubated at 37oC for 24 hours. The suspected colonies were picked up and purified. The pure isolates were identified morphologically and biochemically.
1.2. Methods for identification of suspected Salmonella:
1.2.1. Morphological and biochemical identification:
Dry heat fixed smears of suspected colonies were stained with Gram's stain. The stained smears were examined; revealing the presence of Gramnegative bacilli. The suspected isolates were identified biochemically according to Cruickshank et al., (1975) by applying catalase test, oxidase test and IMViC group of biochemical tests. The identified isolates as Salmonella species were cultivated on triple sugar iron agar (TSI).
1.2.2. Serological identification:
Serological identification of the isolates was carried out according to Cruickshank et al., (1975) at Central Health Laboratories, Ministry of Health and Population. Biochemically identified Salmonella isolates were examined using polyvalent and monovalent O and H Salmonella antisera.
1.3. Confirmation of S. Enteritidis by PCR:
Serologically identified S. Enteritidis isolates were confirmed by PCR using the following primers; Upstream primer '5/ AGG TTC AGG CAG CGG TTA CT /3' and Downstream primer '3/ GGG ACA TTT AGC GTT TCT TG /5' (Olivera et al., 2002). Briefly, genomic DNA was extracted by boiling of bacterial suspension for 10 minutes then centrifugation and the supernatant was used as a template for PCR. The PCR reaction was conducted in a total volume of 50 μl in 200 μl microfuge tube containing 25 μl Master mix, 10 μl template DNA , 4 μl upstream, 4 μl downstream primer and 7 μl nuclease free-water and reaction mixture was overlaid with 50 μl of nuclease free mineral oil to prevent evaporation during thermocycling. Programming of the thermocycler was carried as follow; initial denaturation for 5 minutes at 94oC followed by 35 cycles of denaturation, primer annealing and extension at 94oC for 30 seconds, 60oC for 1 minute, and 72oC for 30 seconds, respectively. At the end, a terminal cycle of extension at 72oC for 10 minutes was applied to ensure that the entire PCR product is double stranded DNA. PCR product was detected by agarose gel electrophoresis using agarose gel 1.5% and staining using ethidium bromide (0.5 μg/ml).
1.4. Plasmid Profiling of S.
Enteritidis (Sambrook et al., 1989): The isolated confirmed S. Enteritidis was tested for presence or absence of plasmid using alkaline lysis method (Sambrook et al., 1989). Briefly, a single bacterial colony which has been grown on LB broth and incubated over night at 37 oC. 1.5 ml then of LB broth culture were poured into a microfuge tube and centrifuged at 12,000 rpm for 30 seconds at 4oC; the supernatant was removed and the pellet was dried. The pellet was resuspended in 150 μL of Tris- EDTA buffer [10 mM Tris chloride (pH 8), 1 mM EDTA (pH 8)] solution by vigorous vortexing and incubation for 5 minutes at room temperature. After 5 minutes, 200 μL of NaOH-SDS (0.2 M NaOH, 1% SDS) solution were added and vortexed then incubated for 5 minutes on ice. After 5 minutes, 150 μL of 3M potassium acetate (pH 4.8) were then added and vortexed for 10 sec and incubated for 5 minutes on ice. Then microfuge tubes were centrifuged again at 12,000 rpm for 5 min at 4°C and the supernatant was precipitated with double volume of ice cold ethanol. Plasmid DNA was loaded onto a .7% agarose gel containing ethidium bromide at final concentration of 0.5 μg /ml and electrophoresed in TBE (Tris Boric acid EDTA) buffer. The plasmid DNA was visualized by placing the gel on a UV transilluminator and the photos were taken by digital camera.
2. Results
2.1. Results of bacteriological
isolation and identification: The totally collected 125 cecal samples that were pre-enriched on tetrathionate broth then cultivated on MacConkey agar medium revealed that among these samples, only 67 of them yield pale colonies with no growth obtained by the other 58 samples (table 1). The recovered suspected colonies were examined microscopically by Gram stain revealing the presence of Gramnegative bacilli then identified biochemically. Those isolates that are identified as catalase +ve, oxidase –ve, indole –ve, methyl red +ve, citrate utilization +ve and lysine decarboxylase +ve; were identified as Salmonella spp. (table 2) and confirmed by cultivation on TSI; producing red slant and yellow butt with H2S production.
Those confirmed Salmonella spp. isolates were serotyped using O and H monovalent specific antisera revealing that only 5 isolates are S. Enteritidis (table 3).
Table(1):Result of growth on MacConkey agar after pre-enrichment on tetrathionate broth indicating that only 67 samples yielded pale colonies among all collected samples.
Table (2): Result of biochemical tests indicating that only 27 among all suspected colonies were identified as Salmonella spp. All of them were sent to the Central Lab of Ministry of Health.
Table (3): Results of serotyping of Salmonella spp. indicating that only 5 isolates are S. Enteritidis.
2.2. Results of PCR and plasmid profile:
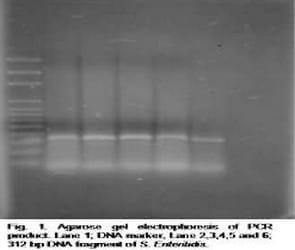
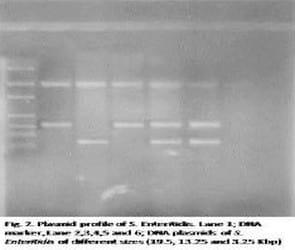
The identified isolates as S. Enteritidis were confirmed by PCR using specific primers revealing the presence of 312 bp specific DNA fragment by all isolates (Fig. 1). DNA plasmids of these isolates were extracted and electrophoresed indicating the presence 19.5, 13.25 and 3.25 Kbp plasmids with three different profiles (Fig. 2).
3. Discussion
Prevention of Salmonella infection is important for poultry and human health. Prevention could be achieved only by good monitoring and screening programs depending on early diagnosis of S. Enteritidis infections birds. (Humbert, et al 1997and Notermans et al., 1997).
Salmonella detection by bacteriological methods usually requires 5-11 days (Humbert et al., 1997) and samples with low numbers of Salmonella cells may give false-negative results.
In our study here, the total number of S. Enteritidis isolated from cecal samples of poultry farms was 4% which agreed with result obtained by Ebel et al., (1992) who recorded S. Enteritidis in 3 % of chicken pooled cloacal samples. contradictory, our finding were not agreed with results obtained by Dreesen et al., (1992) who isolated S. Enteritidis from cloacal samples of laying hens at an incidence of 18.4%. Also, Corkish et al., (1994) reported that 17 % of the flocks were carrier for S. Enteritidis and Neif and Hoop (1998) isolated S. Enteritidis from caecal content of layers with an incidence of 50%.
PCR is a sensitive technique for detection of pathogens and our study confirmed that detection of 312 bp DNA fragment using specific primers is good for detection of S. Enteritidis and this result agreed with results obtained by Oliveira et al., (2002) and Eyigor and Carli (2003) who found that 312 bp notifies the presence of S. Enteritidis DNA.
There are different methods for typing of S. Enteritidis as. Serotyping, phage typing, randomly Amplified Polymorphic DNA, Plasmid Profiling, Restriction Fragment Length Polymorphism and IS200, Ribotyping.
Serotyping depending on O and H antigens which is the one of the oldest methods, it is the commonly used method to differentiate members of the genus Salmonella following the biochemical identification.
Bacterial strains can be typed according to the plasmid content or plasmid profile of each isolate. Plasmids are not considered as a stable strain characteristic as they can be lost or undergo re-arrangement by conjunctive transfer (Liebana, 2002 and Domig et al., 2003) and therefore plasmid profiling is usually used as additional methods for typing.
Disadvantages of plasmid profiling are not always associated with epidemiological identical strains and lost by repeated subculture of bacteria over long period of times. Chadfield et al., (2001). Plasmid profiling was sensitive when it was applied for the typing of S. Typhimurium, S. Virchow and S. Gallinarum strains (Mohan et al., 1994; Liebana, 2002). Controversially; (Adams and Moss 2000) mentioned that Plasmid profiling sometimes met with some success as an epidemiological tool, distinctive and stable method for typing. Plasmid profile of S. Enteritidis isolated in our study indicated the presence of a common heavy plasmid of approximate size of 19.5 Kbp and this agreed with the result of Hegazy (2002). Also, three different plasmid profiles have been identified from the five confirmed S. Enteritidis isolates; profile No 1 having plasmids of molecular size 19.5 Kbp and 13.25 Kbp (lane 1 and 3), profile No 2 having plasmids of molecular size 19.5 and 3.25 Kbp (lane 2) and profile No 3 having plasmids of molecular size 19.5 Kbp , 13.25 Kbp 3,25 Kbp ( lane 4 and 5). The difference noted here in plasmid profiling of the five confirmed S. Enteritidis isolates; indicated that phenotypically similar strains, of S. Enteritidis may be genotypically different. So, plasmid profiling is considered a simple technique that could be used a genotyping method especially in case of epidemiological studies.
4. Conclusion
1- Cecal, liver and spleen samples are a good source for isolation of S. Enteritidis to detect the presence of infection with it inside poultry farms.
2- PCR technique is a sensitive confirmatory method detection of S. Enteritidis.
3- Plasmid profiling is a simple rapid applicable method that could be used as an epidemiological typing method for S. Enteritidis.
5. References.
1. Abd EL-Latif, M.M. 1995. Bacterial causes of lowering fertility, hatchability and early embryonic deaths in balady hatcheries in Dakahlia Governorate. M. V. Sc. Thesis (Bacteriology) Fac. Vet. Zag. Univ.
2. Abou-Zeed, Y.M., Hariharan, H., Poppe, C., Kibenge, F.S. 2000. Characterization of Salmonella isolate from beef, broiler chicken and human sources on Prince Edward Island. Comp. Immunol. Microbiol. Infect. Dis., 23 (4):253-266.
3. Adams, M.S., Moss, M.O. 2000. Food Microbiology 2nd Ed. Royal Society of Chemistry. Thomas Graham House, Science Park, Milton.Road Cambridge CB40WF, UK.
4. Chadfield, M., Skov, M., Christensen, J. Madsen, J. M. and Bisgaard, M. 2001. An epidemiological study of Salmonella enterica serovar 4, 12: b: in broiler chicken in Denmark. Vet. Microbiol. 82, 233- 247.
5. Corkish, J., Davis, R., Wray, C. 1994. Observation on a broiler breeder flock naturally infected with Salmonella Enteritidis phage type4. Vet. Res., 591- 594.
6. Cruickshank, R., Duguid, J.P., Marmion, B.P., Swain, R.H.A. 1975. Medical Microbiology. 12Th Ed the practice of Medical Microbiology.12thEd.ChurchillLivingstone, Edinburgh, and London.
7. Domig, K., Mayer, H., Kneifel, W. 2003. Methods used for the isolation, enumeration, characterization and identification of Enterococcus spp. 2. Phenotypic and genotypic criteria. Int. J. of Food Microbiol. 88: 165-188.
8. Dreesen, D.W., Barnhart, H.M., Bruke, J.L., Chen, T., Johnson, D.C. 1992. Frequency of Salmonella Enteritidis and other Salmonella in the ceca of spent hen at time of slaughter. Avian Dis., 36 (2): 247- 250.
9. Ebel, E.D., David, M.J., Mason, J. 1992. Occurrence of Salmonella Enteritidis in the U.S. commercial egg industry: report on a national spent hen survey. Avian Dis., 36(3): 646-654.
10. Eyigor, A., Carli, K.T. 2003. Rapid detection of Salmonella from poultry by real-time polymerase chain reaction with Fluorescent Hybridization probes. Avian Dis.47 (2):380-6.
11. Hegazy, A.H. 2002. Epidemiological studies on Salmonella with special reference to Salmonella enteritidis. Ph.D. Thesis. Fac. Vet. Med. Alex. University.
12. Humbert, F., Carraminana, J.J., Lalande, F., Salvat, G. 1997. Bacteriological monitoring of Salmonella enteritidis carrier birds after decontamination using enrofloxacin, competitive exclusion and movement of birds. Vet. Rec. 141:297-299.
13. Humphrey, T.J., Williams, A., MeAlpine, K., Lever, M.S., Guard-Petter, J., Cox, J.M. 1996. Isolates of Salmonella enterica enteritidis pt4 with enhanced heat and acid tolerance are more virulent in mice and more invasive in chickens. Epidemiol. Infect., 117(1): 79-88.
14. Liebana, E. 2002. Molecular tools for epidemiological investigation of Salmonella enterica subspecies enterica infections. Research in Vet. Science, 72: 169-175.
15. Mahon, J., Murphy, C.K., Jones, P.W., Barrow, P.A. 1994. Comparison of multiplex PCR and standard bacteriological methods of detecting Salmonella on chicken skin. Lett. Appl. Microbiol., 19(3): 169-172.
16. Nief, V., Hoop, R.K. 1998. Detection of Salmonella Enteritidis in suspected flocks of laying hens. Schweiz. Arch. Tierheilked., 140(2): 70-75.
17. Notermans, S.A. Van de Giessen and Henken, A.M. 1997. Future requirements for diagnosing and monitoring of pathogenic microorganisms in poultry and eggs, p 9-14. In C.J. thorns, and P. Jones(ed), cost action 97 pathogenic microorganism in poultry and eggs.2 monitoring procedures, rapid detection methods and techniques. Office for Official Publication of the European Communities, Luxembourg, Belgium.
18. Oliveira, S.D., Santos, L.R., Schuch, D.M., Silva, A.B., Sale, C.T., Canal, C.W. 2002. Detection and identification of Salmonella from chicken related samples by PCR. Vet. Microbiol. 87(1): 25-35.
19.Sambrook, J., Fritsh, E.F., Maniatis, T. 1989. Molecular cloning a laboratory Manual. 2nd ed., Cold Spring Harbor Laboratory Cold Spring. New York.
20. Stone, G.G., Oberst, R.D., Hays.M., Mcveys, S., Chengappa, M.M. 1994. Detection of Salmonella Serovars from clinical samples by enrichment broth cultivation-PCR Procedure.J. CLin. Microbiol., 32(7):1742-1749.
21. Towner, K., Cockayne, A. 1993. Molecular methods for microbial identification and typing. 1st Ed.
22. Wallance, H.A., June, G., Sherrod, P., Hammack, T.S., Amaguana, R.M. 1999. Salmonella in Food and Drug Administration, Bacteriological Analytical Manual. Tomlison, L.A. (ed).