I. Introduction
Phytase, apart from increasing phosphorus (P) availability from plant ingredients, improves availability of non-P nutrients like Ca, amino acids (AA), and energy (AME) by facilitating the breakdown of phytate P and thus negating its antinutritional effects (Dersjant-Li et al. 2016). Phytase along with carbohydrase enzymes could improve productivity of chickens with a lower feed cost when proper downspec (reduction of nutrient levels in the diet) is applied. It is reported that a novel consensus bacterial 6-phytase may completely replace the inorganic P (equivalent to 0.28% available P) from Mono Calcium Phosphate (MCP) in a broiler diet whilst maintaining performance and bone mineralization (Marchal et al., 2021). Bacillus based probiotics influence gastrointestinal tract (GIT) microbiota and reduce the numbers of Avian Pathogenic E. coli in the GIT of broiler chickens (Pedroso et al., 2016). These probiotics work by outcompeting the non-beneficial bacteria while encouraging the growth of the beneficial ones and aiding development of the immune system (Ouwehand et al., 2010, Bento et al., 2013, Wealleans et al., 2017) with a more conspicuous effect being observed in the presence of enteric challenges (Dersjant-Li et al. 2016).
II. Methods
Five hundred male Vencobb 430 chicks (Venkateshwara Hatcheries P Ltd., Pune, India) procured from a local hatchery were assigned to one of five treatment groups following a completely randomized design. The chicks were placed on litter composed of wood shavings and paddy straw in pens (1.2 m x 1.2 m). Each pen had 10 chicks at the beginning and there were 10 such pens allocated randomly to each of the treatment groups (n = 100 per treatment). The birds received feed within 12 h of hatch and were subsequently fed with starter (1-14 d) and grower (15-28 d) feed as crumbles and finisher (29-42 d) feed as pellets. The lighting program included 24 h light during the first week followed by 20 h light from the second week until harvest. Room temperature was maintained around 30°C with the help of electrical brooders during the first 2 weeks of the experiment.
The experiment involved feeding the birds with a control diet which was formulated to meet the requirements of the breed and was devoid of any gut acting growth promoter, antibiotics and anti-coccidial agents, a negative control (NC) diet which had a nutrient downspec of 0.199% Ca, 0.194% available P, 110 kcal energy and 0.704% crude protein (CP) according to the full matrix recommendations for the consensus bacterial 6-phytase (1000 FTU/kg - Axtra PHY® GOLD) and xylanase, amylase and protease (2000 U/kg xylanase, 200U/kg α-amylase and 4000 U/kg protease - Axtra XAP®)) combination (E) that would be supplemented to the NC diets. Apart from the aforementioned enzyme combination, the NC diet was further supplemented with a multi-strain Bacillus probiotic (PB - Enviva PRO®) supplying 150,000 CFU/g (Colony Forming Units) of the product (NC+E+PB). The group fed with the NC diet was challenged enterically as described below (C-NC) and the effect of the enzyme-probiotic combination was ascertained by supplementing the C-NC group with the said combination (C-NC+E+PB).
The challenge model consisted of a primary inoculation with avian pathogenic E. coli (APEC) at 11-12 d of age, followed by a mixed Eimeria infection (consisting of 8000 sporulated oocysts of E. acervulina, E. maxima and E. tenella per chicken administered through an oral gavage) and finally Clostridium perfringens (ATCC 13124) infection given during 18 -20 d. The APEC was isolated from field infections, cultured and confirmed by the presence of papC, iucD and femC genes (Paixao et al., 2016; Zakariazadeh et al., 2019; Mohamed et al., 2018; Subedi et al., 2018;) by targeting the specific 16s rRNA through polymerase chain reaction (Sobur et al., 2019). The Eimeria oocysts were collected from field infections and were maintained by periodic passage through coccidia-free chicks and sporulated in 2% potassium dichromate solution.
Body weight (BW) was measured at 0 and 42 d. Average daily gain (ADG) in BW and feed conversion ratio (FCR) which was calculated as a ratio of total feed intake (FI) to gain in BW were determined along with liveability and European productivity index (EPI) for the period of 1-42 d. Whole blood samples were collected at 25 d and the serum was analysed for anticlostridium alpha toxin antibody (CP-α-T-Ab). One bird, selected randomly from each of the replicates under each treatment, was killed humanely and mucosal scrapings collected from across the length of the small intestine were analysed for alpha-1-acid glycoprotein (α-1-AGP) and secretory immunoglobulin A (SIgA) by enzyme linked immune sorbent assays employing commercially available kits specific for chickens. Pens were the experimental units for the performance traits while individual observations were considered as experimental units for analysis of biomarkers. Statistical differences between treatments were determined using ANOVA and Tukey means separation (JMP, SAS software).
III. Results
Data in Table 1 indicates that the nutrient downspec caused reduced BW and inferior FCR (P = 0.0001) in the NC group. Induction of dysbacteriosis further reduced BW and deteriorated FCR (P < 0.05) in the C-NC group. Cumulative FI increased with nutrient down spec in NC diet (P = 0.0001) while dysbacteriosis decreased FI (P < 0.05). Enzyme-probiotic combination restored BW to the level obtained with the Control group and resulted numerically better FCR across all the treatments. The C-NC+PB group performed better than the C-NC group in terms of BW and FCR. Liveability was lower (P = 0.0001) in the C-NC group suggesting conspicuous effect of dysbacteriosis in this group. Considering EPI as the index to overall flock performance, it was concluded that in the absence of any enteric challenge, it is possible to sustain performance with a down spec diets supplemented with consensus bacterial 6-phytase and xylanase/amylase/protease combination. Supplementation of Bacillus probiotic with these enzymes might yield better BW and FCR. In the presence of dysbacteriosis, a visible reversal in performance drop is possible when Phytase (Consensus bacterial 6-Phytase) and combination of Xylanase, Amylase and Protease enzymes (XAP) were supplemented along with the probiotics (PRO).
Table 1 - Performance traits during 1-42 d and concentration of immune biomarkers in serum and mucosal scrapping (25 d) of the experimental broiler chickens 1
Induction of dysbacteriosis increased the activity of α-1-AGP in the mucosal scrapings (P = 0.0001) of the C-NC group. A marginal rise in α-1-AGP activity in the mucosal scrapings of NC group suggests some proinflammatory changes taking place due to feeding of the unsupplemented diet which was richer in phytate P. Activity of SIgA was higher (P = 0.001) in the NC + PB group which might be to an immune stimulatory effect of probiotic a comparatively higher SIgA activity in the C-NC group might be attributed to the hist defence mechanism.
IV. Discussion
The result of the present study suggests that the enzyme-probiotic combination was useful in sustaining performance of the birds not only with nutrient down spec but also in the presence of dysbacteriosis challenge. The data further suggests that the effects of either of these challenges became more conspicuous as the birds grew older and the enzyme/probiotic combination required some time to influence the performance traits since they work more by modulating the GIT microbiota and nutrient turnover patterns rather than by yielding a direct antibacterial effect. Up to 14-d (data not shown) nutrient down spec according to the enzyme matrix may not make the diet too marginal for growth of the young chicks and supplementation of the probiotic with the enzymes not only covered the nutrient down-spec but improved BW beyond that of the Control group. Thus, these findings validated the matrix contributions from Phytase and XAP in combination with the probiotic. Decline in BW in the C-NC group was expected and improvement in performance with probiotic in the C-NC+PB group suggested a beneficial effect of the PB in reversing the negative effect of dysbacteriosis. Concentration of α-1-AGP in the mucosal scrapings indicated that the enzyme-probiotic combination probably sequestered the dysbacteriosis induced pro-inflammatory changes in the GIT. Circulatory CPα-T-Ab concentration, which was below the detectable limit, was rather predictable since without any clostridial challenge it was unlikely that the antibody would be found in the circulation. It is interesting to note the sharp decline in the level of the same antibody in the challenged C-NC+PB group which is suggestive of the beneficial effect of enzyme-probiotic combination and validates the concept which advocates the possibility of maintaining the GIT health with such “green” approaches without AGPs in food animals.
V. Conclusions
It was concluded that with phytase combined with a xylanase, amylase, protease combination, it may be possible to sustain performance of broiler chickens with diets diluted in terms of nutrients and this strategy should provide scope to reduce feed cost by sparing costly ingredients like oil and protein meals in formulations. The concept of maintaining GIT health with an enzyme-probiotic combination is supported by the results of this study which shows that even in the absence of a conventional AGP, broiler chickens retained their performance with dietary supplementation of the enzyme-probiotic combination while being exposed to a mixed enteric challenge including dysbacteriosis and Clostridium perfringens induced necrotic enteritis.
Presented at the 33th Annual Australian Poultry Science Symposium 2022. For information on the next edition, click here. 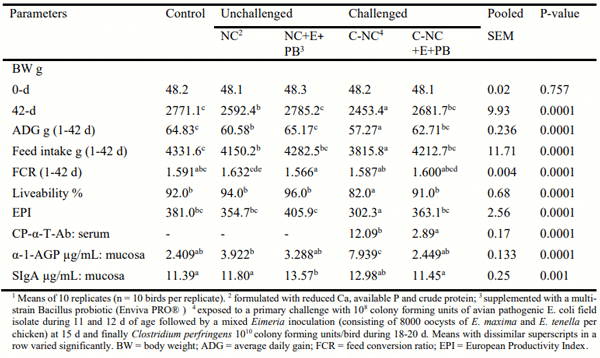











.jpg&w=3840&q=75)