The Effect of Digesta Viscosity in Broiler Chickens
The Effect of Digesta Viscosity on Transit Times and Gut Motility in Broiler Chickens
Published: April 13, 2012
By: Adam Sacranie (Nutreco), B. Svihus (Norwegian University of Life Sciences) and Assoc. Prof. Paul Iji (University of New England)
The hypothesis that an increase in luminal viscosity would result in reductions in performance parameters, digesta passage rate and frequency of reverse peristalsis was tested. Chickens were fed diets containing varying quantities of guar gum, alginic acid or corn starch to influence digesta viscosity. The two guar gum treatments yielded the highest intestinal viscosities mirrored by very high feed conversion ratios, low starch digestibility and, for birds exposed to the highest level of guar gum, very low weight gain compared to the control birds. An association between a rise in ileal viscosity and low transit times was recorded; however at the highest viscosity, transit rates were significantly less than the control. Cloacally administered Cr-EDTA was retrieved from the gizzards of birds in all treatments. The marker was recovered in greater amounts in birds exhibiting lower ileal viscosities, with the exception of birds from the high level guar gum group, displaying faster passage rates and lower ileal viscosities.
I INTRODUCTION
The presence of viscous chyme in the lumen, due to diets high in soluble non starch polysaccharide (NSPs), results in longer retention times than diets low in NSPs or containing exogenous enzymes (Almirall and Esteve-Garcia, 1994; van der Klis and van Voorst, 1993). Variations in retention times may influence the performance of the broiler by affecting the time of exposure of digesta to the digestive enzymes and absorptive sites, as well as shaping the microflora composition present in the gastrointestinal tract GIT (Choct, et al., 1996).
The occurrence of reverse peristalsis will influence transit times. Reverse peristalsis, or reflux, has been identified in fasted and fed chickens, and is viewed as a mechanism by which the bird can maximise nutrient availability in the feed by prolonging the exposure of digesta to the digestive and absorptive sites along the GIT (Clench and Mathias, 1992; Clench and Mathias, 1995; Dziuk and Duke, 1972; Sacranie, et al., 2008). It is not known how a change in the flow dynamics of digesta, by an increase in viscosity, might affect its occurrence.
The following trial was conducted to investigate the inter-relationships between, intestinal viscosity, digesta passage rates and the occurrence of reverse peristalsis.
II MATERIALS AND METHODS
Two hundred and ten day-old male Cobb broiler chicks (Baiada Poultry Pty. Ltd., Kootingal, NSW, Australia), balanced for weight, were randomly divided into 7 groups and assigned to a separate level of a multi-brooder. A mash commercial diet plus one of three carbohydrates, alginic acid (A, a low viscosity NSP, 1.38 cP in 1% solution), guar gum (G, galacto-mannan, 4000 cP in 1% solution) and corn starch (S, approximately 73% amylopectin and 27% amylase, 340 cP in 1% solution); at either a 1 or 2 % dilution, was presented to six groups. The control birds received the commercial diet without a carbohydrate additive. An initial room temperature of 34o C was maintained for the first 5 days and gradually decreased to 21oC by 21 days of age. At 21 days of age, 12 birds from each group were moved and placed in individual cages for transit rate estimation and motility measurements. The remaining birds were weighed and randomly assigned to slide-in cages, 3 birds per cage. Birds had ad libitum access to both feed and water. At 27 days of age, the broilers were given access to feed for one hour. After feeding, six birds from each treatment group were weighed and a gelatine capsule containing approximately 0.15 g ferric oxide was placed on the oro-pharynx for spontaneous deglutition, without any previous habituation. The time was recorded in minutes from administration of the marker until first appearance in the faeces. At 28 days of age, 2 birds from each cage across all seven treatments were euthanized by CO2 asphyxiation. Birds were dissected; the weights of the empty gizzard, pancreas and empty small intestine were recorded. Contents of the ileum were collected for starch digestibility and viscosity analysis.
At 30 days of age, one individually caged bird from each of the treatment groups was denied access to feed for one hour. After fasting, the chickens received a 1 mL injection of Cr-EDTA (2.66 mg of Cr) into their cloaca via a crop needle. Birds were placed back in their cages and given access to feed. After two hours post injection, the chickens were euthanised by CO2 asphyxiation, dissected and digesta samples quantitatively collected from the gizzard, duodenum, jejunum and ileum. The process was repeated across replicates and treatments.
Digesta samples were weighed, freeze dried, dry matter (DM) calculated, digested in perchloric acid and hydrogen peroxide, and measured for mineral content using an inductively coupled plasma spectrophotometer (Binnerts et al., 1968). Total Cr in each section was calculated by multiplying the DM of a given section by the concentration of Cr recovered. The seven treatments were analysed using one way ANOVA, means were separated using Fisher´s test at p≤0.05 significance level.
III RESULTS
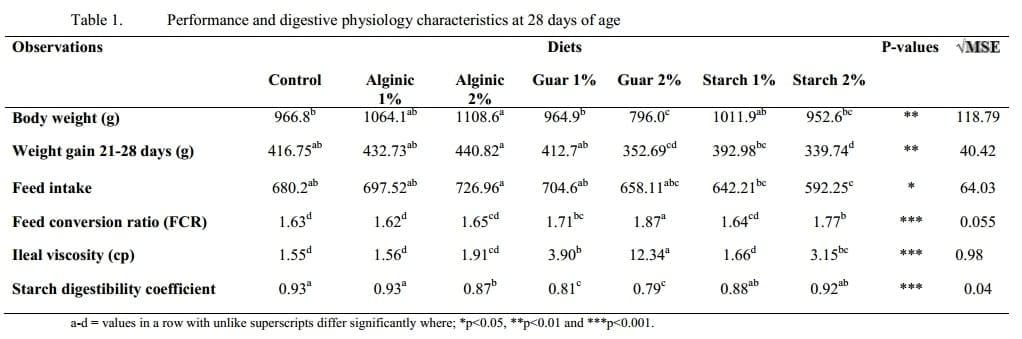
The least desirable feed conversion ratio (FCR) was observed in birds from the G-2% treatment group, at 1.87, the average FCR was significantly higher (p<0.001) compared to chickens from the other six treatments, as shown in Table 1. The A-1% and C broilers displayed the lowest (p<0.001) FCR, 1.62 and 1.63, respectively.
Analysis of the ileal contents of birds from the G-2% group revealed a strong effect on viscosity, with significantly higher (p<0.001) values (12.34 cp) than those recorded in the digesta of broilers from the other six treatments. Chickens fed the G-1% and S-2% diets had considerably higher (p<0.001) readings for ileal viscosity than the control (the least viscous). Starch was most readily (p<0.001) utilised in birds from the C, A-1%, S-1% and S-2% treatment groups. Chickens raised on the G-1% and G-2% feeds yielded the lowest (p<0.001) digestibility values for starch (0.81 and 0.79, respectively).
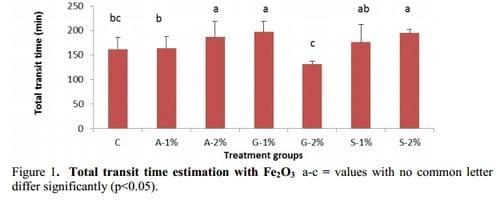
The longest retention time, 197 minutes, was recorded in the G-1% birds, significantly higher (p<0.05) than the C, A-1% and G-2% and comparable to the S-1%, S-2%, and A-2% treatment groups, as shown in Figure 1. The quickest rate of passage of the visual marker was observed in birds from the G-2% group, at 131 minutes, it was considerably faster (p<0.05) than birds from the other six treatment groups.
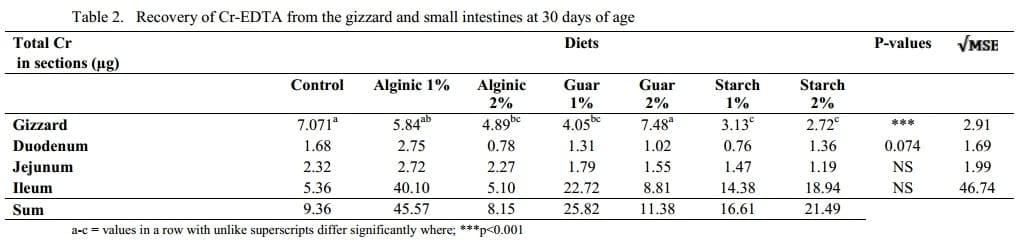
The soluble marker, Cr-EDTA, was retrieved from the gizzard contents of chickens from all seven treatment groups, as shown in Table 2. The highest (p<0.001) amount of Cr in the gizzard chyme was observed in birds from the G-2% group, comparable to levels in C, A-1% and A-2% treatments. The lowest (p<0.001) amounts of Cr in the gizzard digesta were recorded in broilers from the S-1% and S-2% groups. Compared to gizzard contents, levels of Cr decreased in the duodenum of birds across all the treatments. There was a tendency (p=0.074) for higher levels of Cr in the duodenum of birds from the A-1% group, similar to those observed in birds from the C treatment.
IV DISCUSSION
In general a negative relationship was observed between viscosity and performance parameters. The two guar gum treatments yielded the highest intestinal viscosities; the dramatic rise from a 1 to 2 % tended to initiate exponential rises in viscosity with increases in guar gum concentration. The high ileal viscosities in birds from these two treatments were mirrored by very high feed conversion ratios, low starch digestibility and for birds exposed to the highest level of guar gum; very low weight gain compared to the control birds in concurrence with previous studies (Bedford and Classen, 1992; Choct and Annison, 1992).
A longer retention time of digesta is associated with high intestinal viscosities. Slow moving intestinal contents provide a greater opportunity for microbial proliferation, poaching nutrients that could otherwise be used for growth (Choct, et al., 1996). There was an association between higher ileal viscosities and slower rate of passage of Fe2O3.
The transit rates for the control and A-1% birds were in the range of expectation for broiler chickens exposed to low-viscous diets, at that age (Danicke, et al., 1999; Hughes, 2008). The longest retention times were observed in chickens from the A-2%, G-1% and S-1% treatment groups.
The results from the A-2% and S-1% treatment groups, in this study, suggest a possible positive relationship between a longer retention time of digesta in the lumen and performance. As already stated, starch digestibility was slightly lower in birds from both groups (significantly so, in the A-2% group) compared to chickens raised on the commercial diet, however, FCR remained relatively unaffected. In addition chickens from these two treatments had the highest body weights. While starch digestion was obviously retarded by the slight rise in viscosity, it was perhaps not enough to hinder absorption of its digestion products and other nutrients.
Interestingly, the G-2% birds displayed a significantly increased rate of passage of the marker, compared to chickens from the other six treatments, even though they possessed the highest ileal viscosity. Digesta are propelled along the intestines by migrating myoelectric complexes in the smooth muscles of the intestines. Smooth muscle contraction on the orad side induces a rise in intraluminal pressure which is then absorbed by the corresponding relaxation of muscle on the aborad side, as the viscosity of the digesta increases so does the pressure required for propulsion (Lentle and Janssen, 2008). The pressure required to transport highly viscous digesta, as observed in the lumen of G-2% birds, may be reduced by the formation of a less viscous peripheral layer close to the mucosal surface if the intestines, this reduces friction and promotes flow (Lentle and Janssen, 2008). It is possible that the luminal contents of birds from the G-2% treatment, characterised by a highly viscous solid suspension fraction and a less viscous fraction containing the visual marker. The increased pressure of the smooth muscular contractions may have been partly absorbed by the fluid fraction; inducing a fast flow of this fraction and resulting in the rapid transit rates recorded in these birds, while ineffective in displacing the highly viscous solid suspension fraction.
The highest recovery of Cr was recorded in A-1% birds, equivalent to 1.7% of the total Cr introduced to each chicken, compared to 0.3% in control birds. Previous research conducted by the same group observed detectable levels in the crop of birds and that the majority of cloacally administered Cr-EDTA flows immediately into the ceca, and in addition, refluxed Cr from proximal sites collects in the ceca over time (Sacranie, et al., 2008). In the current trial samples for Cr analysis were not collected from the ceca or crops of birds or crop, due to laboratory limitations most likely contributing to the low total recoveries. The Cr results from the gizzard suggest an association with the transit times recorded in birds from this trial. The highest levels of Cr recovered from the DM of gizzard contents and the fastest rate of passage of the Fe3O2 through the GIT, was observed in the G-2%, C and A-1% birds, in that order. A very fast passage rate through the lumen as seen in the G-2% birds may reduce satiety, triggering a reverse motility response similar to what has been observed in fasted chickens, and often attributed by unique motility patterns referred to as rhythmic oscillating complexes or ROCs (Clench and Mathias, 1992; Clench and Mathias, 1995; Jimenez, et al., 1994; Sacranie, et al., 2008). The results from the G-2 % birds could represent an adjustment to the normal motility conditions along the GIT as a result of the fast transit times. Under these conditions, the occurrence of reflux may be up regulated.
REFERENCES
Almirall M, Esteve-Garcia E (1994) Poultry Science 73, 1433-1440.
Bedford MR, Classen HL (1992) Journal of Nutrition 122, 560-569.
Binnerts WT, Vantkloo.At, Frens AM (1968) Veterinary Record 82, 470-&.
Choct M, Annison EF(1992) The Journal of Nutrition 122, 2457-2465.
Choct M, Hughes RJ, Wang J, Bedford MR, Morgan AJ, Annison G (1996) British Poultry Science 37,609-621.
Clench MH, Mathias JR (1992) American Journal of Physiology 262, G498-G504.
Clench MH, Mathias JR (1995) Condor 97, 1041-1047.
Danicke S, Vahjen W, Simon O, Jeroch H (1999) Poultry Science 78, 1292-1299.
Dziuk HE, Duke GE (1972) American Journal of Physiology 222:159-166.
Hughes RJ (2008) British Poultry Science 49, 716-720.
Jimenez M, Martinez V, Rodriguezmembrilla A, Rodriguezsinovas A, Gonalons E, Vergara P (1994) American Journal of Physiology 266, G585-G595.
Lentle R, Janssen P (2008) Journal of Comparative Physiology B: Biochemical, Systemic, and Environmental Physiology 178, 673-690.
Sacranie A, Iji PA, Mikkelsen LL, Choct M (2008) Proc. The 23rd World´s Poultry Congress, June/July, Brisbane. van der Klis JD, van Voorst A(1993) Poultry Science 72, 503-512.
Bedford MR, Classen HL (1992) Journal of Nutrition 122, 560-569.
Binnerts WT, Vantkloo.At, Frens AM (1968) Veterinary Record 82, 470-&.
Choct M, Annison EF(1992) The Journal of Nutrition 122, 2457-2465.
Choct M, Hughes RJ, Wang J, Bedford MR, Morgan AJ, Annison G (1996) British Poultry Science 37,609-621.
Clench MH, Mathias JR (1992) American Journal of Physiology 262, G498-G504.
Clench MH, Mathias JR (1995) Condor 97, 1041-1047.
Danicke S, Vahjen W, Simon O, Jeroch H (1999) Poultry Science 78, 1292-1299.
Dziuk HE, Duke GE (1972) American Journal of Physiology 222:159-166.
Hughes RJ (2008) British Poultry Science 49, 716-720.
Jimenez M, Martinez V, Rodriguezmembrilla A, Rodriguezsinovas A, Gonalons E, Vergara P (1994) American Journal of Physiology 266, G585-G595.
Lentle R, Janssen P (2008) Journal of Comparative Physiology B: Biochemical, Systemic, and Environmental Physiology 178, 673-690.
Sacranie A, Iji PA, Mikkelsen LL, Choct M (2008) Proc. The 23rd World´s Poultry Congress, June/July, Brisbane. van der Klis JD, van Voorst A(1993) Poultry Science 72, 503-512.
This paper was presented at the 23rd Annual Australian Poultry Science Symposium, in Sidney, New South Wales from February 19-22, 2012. Engormix.com thanks the organizing committee and the authors for this contribution.
Content from the event:
Related topics:
Authors:

Nutreco
Recommend
Comment
Share
Nutreco
12 de junio de 2012
Thanks Ganesh,
it is, indeed, possible for microbial species to be relocated, we have demonstrated this, in as yet, unpublished data.
Adam
Recommend
Reply
Guybro Chemical
21 de mayo de 2012
Nice article about Gut Motility in Broiler Chickens !
Will reverse peristalsis in lower GIT also lead to reflux of pathogen?
Thanks !
Recommend
Reply

Would you like to discuss another topic? Create a new post to engage with experts in the community.
Featured users in Poultry Industry

Shivaram Rao
Pilgrim´s
PhD Director Principal de Nutrición y Servicios Técnicos de Pilgrim’s Pride Corporation
United States
United States