I. INTRODUCTION
Maintenance of intestinal integrity is of fundamental importance to the overall health and growth performance of the bird. Disturbances in the homeostasis of the gastrointestinal tract can result in inflammatory process, loss of intestinal integrity, low nutrient digestibility and poor growth performance. In a situation of intestinal inflammation, pro-inflammatory interleukins such as interleukin-12 are produced with the objective to defending the host from any aggression. However, pro-inflammatory interleukins have a high potential to reduces host homeostasis, causing fever, muscle catabolism and disruption of the intestinal tight junction proteins (Al-Sadi et al., 2013). Claudin-1 and occludin are tight junction proteins that help seal the paracellular space between enterocytes, preventing the translocation of microorganisms from the intestinal lumen into the bloodstream. In addition, there is Mucin-2 (MUC2), the main mucin produced by the Goblet cells, that prevents microbial adhesion to the mucosa. Thus, the higher the intestinal expression of MUC2, claudin-1 and occludin, the better the intestinal integrity. For these reasons, unnecessary overstimulation of the inflammatory leading to a low intestinal health response may negatively affect growth performance and should be alleviated.
A common practice to improve birds’ intestinal health has been the use of feed additives. In AGP-free poultry production, protected OA and EO are strong tools to maintain intestinal homeostasis (Wang et al., 2019). Organic acids and EO act through different modes of action, however when combined, may have a synergistic effect on the intestinal health and growth performance by modulating the microbiota (Adewole et al., 2021) or signaling molecules that influence diverse regulatory functions on host’s physiology, metabolism regulation, inflammation, and immunity (D´Aquila et al., 2020). In addition, the use of protective technology allows these compounds to be slowly released along the digestive tract and reach the posterior portion, where most of the pathogenic bacteria is present. Therefore, the objective of the study was to evaluate the effects of a blend of a protected organic acid + essential oils on growth performance, nutrient digestibility, intestinal health, and immune response of broiler chickens undergoing an intestinal challenge while comparing it to an AGP.
II. METHOD
A total of 1,080 1-day-old Cobb 500 male broiler chicks (body weight: 43 ± 1 g) were randomly distributed in four experimental treatments (10 replicates with 27 birds/each) for 42 days, as follows: T1 – unchallenged control (UC), T2 – challenged control (CC), T3 – challenged supplemented with antibiotic growth promoter (AGP, Enramycin at 10 g/t) and, T4 – challenged and supplemented with a blend of protected organic acids + essential oils [P(OA+EO) at 300 g/t, Jefo Nutrition Inc.]. The P(OA+EO) consists of fumaric, sorbic, malic, and citric acids and thymol, vanillin, and eugenol. Except the NC group, all birds were challenged at 1 day, via individual oral gavage with a commercial coccidiosis vaccine at 10× the regular dose (Bio-Coccivet live vaccine, containing E. acervuline, E. brunetti, E. maxima, E. necatrix, E. praecox, E. tenella, and E. mitis; Biovet Vaxxinova). At 11, 12, and 13 days of age, birds were individually orally gavaged with 1 mL/bird of Clostridium perfringens (108 CFU/bird). All procedures were approved by the Ethics and Research Committee of the Federal University of Santa Maria, Santa Maria, Brazil (number 5404280717).
Body weight (BW), feed intake, body weight gain (BWG), and feed conversion ratio (FCR) were determined by week. Mortality was recorded daily. On day 17 of age, one bird/pen (n = 20 birds/treatment) was orally gavaged with 2.2 mg/bird of systemic fluorescein isothiocyanate-dextran (FITC-d). Blood samples were collected 1 h after gavage to detect FITC-d level in serum. At 21 days, mucosa samples of one bird/pen from the middle of jejunum were collected to evaluate the following markers: mucin-2 (MUC2), claudin-1, occludin and, pro-inflammatory interleukin-12 (IL-12) mRNA expression. Macroscopic (3 birds/replicate, n = 30 birds/treatment) and histological (2 birds/replicate, n = 20 birds/treatment) intestinal alterations were evaluated by a blinded assessor using the I See Inside methodology (ISI®). In this methodology, an impact factor (IF) ranging from 1 to 3 is defined for each alteration in macro/histologic analysis according to the reduction of the organ’s functional capacity, where 3 is the most significant alteration for the organ function. In addition, the intensity and extension of each alteration are evaluated and a score from 0 to 3 is assigned, 0 being the absence of lesion. To reach the final value of the ISI® index, the IF of each alteration is multiplied by its respective score number and the results of each individual alteration are summed (Kraieski et al., 2016). A low ISI score represents better intestinal health (Belote et al., 2019). To determine ileal digestibility, digesta (4 birds/replicate = 40 birds/treatment) was collected from the distal two-thirds of the ileum. Celite at 1% was used as an indigestible marker in the starter diets and this diet was provided 48h prior to ileal digesta collection. The parametric data were subjected to analysis of variance (ANOVA) using the GLM procedure of SAS Institute. Means were analyzed by Fisher LSD. The non-parametric data were submitted to the Kruskal-Wallis. Significance was accepted at P < 0.05 and tendency at P < 0.10.
III. RESULTS
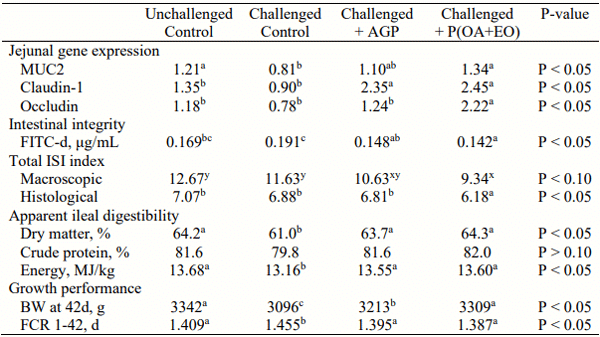
Broilers on P(OA+EO) had better intestinal barrier integrity due to the higher MUC2, claudin1 and occludin gene expression (P < 0.05) and lower levels of serum FITC-d (P < 0.05), compared to CC group (Table 1). Still compared to CC group, broilers on P(OA+EO) had better intestinal health due to the lower total histologic ileal ISI index (P < 0.05; Table 1) and tendency towards a lower macroscopic ISI index (P < 0.10). Broilers on P(OA+EO) had higher dry matter (3.3%) and IDE (0.44 MJ/kg) compared to CC group, and no difference compared to AGP group (P < 0.05; Table 1).
Table 1 - Jejunal gene expression of intestinal biomarkers, serum FITC-d, ISI® index, apparent ileal digestibility and growth performance of broilers undergoing an intestinal challenge.
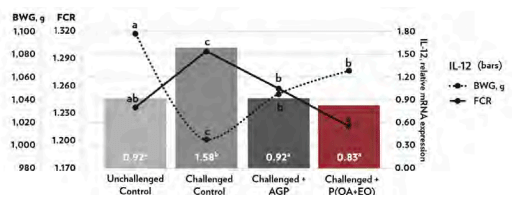
At 21 days old, broilers on CC group had an overstimulation of the inflammatory response (higher IL-12) that resulted in lower BWG and higher FCR (P < 0.05; Figure 1). Proinflammatory IL-12 increases during dysbiosis and has a high potential to disrupt host homeostasis and negatively affect growth performance. Meanwhile, compared to the CC groups, P(OA+EO) was able to alleviate the inflammatory response (lower IL-12) and resulted in a superior growth performance. Furthermore, P(OA+EO) provided a similar response to the AGP group for the pro-inflammatory response while having a lower FCR. At 42 days, the reduced growth performance of the CC compared to UC group (P < 0.05; Table 1) show that the applied challenge model was efficient. Broilers on P(OA+EO) had BW and FCR (1-42 d) similar to the UC group and even had 2.9% higher BW than birds on AGP group (P < 0.05; Table 1).
Figure 1 - Jejunal gene mRNA expression of IL-12, BWG (dotted black line) and FCR (straight black line) on 21-day-old broiler chickens undergoing an intestinal challenge (abcP < 0.05).
IV. DISCUSSION
The intestinal challenge model was efficient to cause a disturbance in the intestinal homeostasis negatively affecting intestinal health, nutrient digestibility and, consequently, the growth performance of broiler chickens. The higher ISI scores, FITC-d serum level and proinflammatory IL-12 expression combined with the lower expression of the tight junction proteins and lower nutrient digestibility observed in CC group justify the poor growth performance of these birds. However, challenged broilers fed diets supplemented with P(OA+EO) had better growth performance, which is in line with the results of better intestinal barrier integrity, nutrient digestibility and health status, the latter being observed through lower ISI scores. Belote et al. (2019) demonstrated that the ISI histological analysis has a strong correlation with growth performance, and that the higher the ISI score is, the poorer the performance of the broiler chickens.
The reduced ISI scores observed in broilers fed P(OA+EO) can also be an indication of a lower demand for mucosal renovation, which may explain the higher expression of MUC2 in this group. Additionally, FITC-d blood levels were lower for broilers on P(OA+EO) group, indicating that even under a dysbiosis model, their intestinal barrier seemed to be less compromised, suggesting a low intestinal leakage and less chance of bacterial and toxins translocation from intestinal lumen to bloodstream. In conclusion, the blend of protected organic acids + essential oils evaluated showed better or similar effects to an AGP in improving intestinal health, nutrient and energy digestibility and modulated the immune response to benefit the growth performance of broiler chickens undergoing an intestinal challenge.
Presented at the 34th Annual Australian Poultry Science Symposium 2023. For information on the next edition, click here. 













.jpg&w=3840&q=75)