INTRODUCTION
Genetic selection programs emphasizing broiler growth, breast muscle deposition, and feed efficiency traits over the past 30 yr have led to a rate of change that has not slowed (Havenstein et al., 2003a,b). Photostimulation (PS) age, feed allocation, and BW profile of the maturing pullet can all effect nutrient partitioning between growth and ovary development (Robinson et al., 2007). Renema et al. (2007b) reported that, as a percentage of BW, Ross 708 pullets had heavier breast muscle and less fat pad compared with Ross 508 and Hubbard Hi-Y birds at sexual maturity. Increased breast muscle weight accompanied by decreased fat pad deposition in current broiler breeder pullets at sexual maturity age could be shifting more energy toward breast muscle maintenance with less energy available for early egg production.
The continued shift in broiler breeder body composition may affect the ability of the pullet to respond to PS. Robinson et al. (1996) examined the effects of PS ages, ranging from 17 to 23 wk of age, and suggested that, though delaying PS delayed the age at first oviposition, the rate at which sexual maturity occurred was more rapid. Though later reports concurred with these results (Renema et al., 2001b, 2007b; Ciacciariello and Gous, 2005b), these experiments generally included only 2 PS ages and primarily examined feeding treatment affects. In a series of experiments examining a combination of PS ages and BW targets, Lewis et al. (2007) identified pullet BW as a primary driver in the responses measured. However, Renema et al. (2007b) indicated that BW effects differed based on age in their comparison of 4 pullet target BW profiles. They reported that lighter pullets matured 17 d after heavier birds when pullets were PS at 18 wk of age, whereas birds of all BW profiles matured at a similar time when PS was delayed until 22 wk of age. Lewis et al. (2007) suggested that heavier pullets become photosensitive at a younger age, allowing them to respond to PS earlier. These birds will be at a more advanced state of physical and physiological maturity at the point of PS.
It is possible that pullet weight alone may account for all of the maturational differences noted in previous studies in this research area. We theorized that the changes in broiler breeder growth potential and carcass composition since the work of Robinson et al. (1996) were large enough to necessitate a new evaluation of the response of broiler breeder pullets to a large range of PS ages. The objective of the current experiment was to examine effects of PS ages ranging from 17 to 23 wk of age on sexual maturity age, carcass and ovarian morphology, and egg weight corrected by BW at sexual maturity in broiler breeders feed restricted starting at 1 or 3 wk of age. In the last 20 yr, PS ages in North America have shifted later. This examination of PS effects on sexual development of current stocks compared with stocks in previous publications will help to characterize change in sensitivity to PS and whether this is affecting the sexual development of broiler breeders.
MATERIALS AND METHODS
The birds studied in this project were managed according to the Guide to the Care and Use of Experimental Animals (Canadian Council on Animal Care,1984). The experimental protocol was approved by the University of Alberta’s Livestock Animal Care and Use Committee.
Experimental Design
A 2 × 4 factorial experiment was conducted with 2 periods of initial full feeding and 4 PS ages. In 4 replicate environmental rooms per treatment, ad libitum access to feed was provided from the time of placement for either 1 (AL1) or 3 wk (AL3) of age, followed by a quantitative feed restriction program. The subsequent target BW profiles converged by 10 wk of age (Pishnamazi et al., 2008). Results of initial duration of full feeding on growth and conformation traits from 0 to 16 wk were presented elsewhere (Pishnamazi et al., 2008). Birds were randomly assigned to individual cages in the 8 chambers with equal numbers of AL1 and AL3 birds at 16 wk of age and PS at 17, 19, 21, or 23 wk of age (PS17, PS19, PS21, and PS23, respectively) with 2 rooms per PS treatment.
Stocks and Management
Seven hundred twenty Ross 308 breeder pullets were assigned to the growth phase of the project, with 128 birds preselected from hatch continuing in the current study. The environmental rooms (3.85 × 4.85 m) were light-tight and had independent temperature controls that held temperature variation among rooms to less than 0.5°C. Rooms were held at 21°C for the duration of the study. Between 3 and 16 wk of age, feed was restricted using a 5:2 system (5 feed days:2 nonfeed days per week). Group BW were obtained weekly from each pen until 3 wk of age and then twice per week thereafter. Pen weights were used to determine feed allocation needed to maintain the target BW growth profile. Feed was provided in mash form with standard starter (0–21 d of age) and grower (21 d to sexual maturity) diets (Pishnamazi et al., 2008), and water was available for ad libitum consumption.
At 16 wk of age, the 128 pullets (64 from each feeding treatment) allocated to this phase of the experiment were randomly allocated to individual cages (30 × 46 cm) housed in the same environmental rooms birds were reared in (8 birds per feeding treatment or room). Each of the 16 laying cages in the portable cage unit had an individual feeder and incandescent light bulb (70 lx). From 16 wk of age birds were fed on a daily basis. Birds were individually weighed weekly and feed allocation was determined once per week on a PS age basis to maintain birds of all treatments on a com-mon BW target profile (Table 1). The lighting program consisted of 24 h of light for the first 3 d, followed by 8L:16D, which was maintained until 17, 19, 21, or 23 wk of age, when the light period was increased to 14 h in a 14L:10D program.
Traits Measured
Pullets were individually weighed weekly from the point they were moved to laying cages. Age at sexual maturity was defined as age at first oviposition. The time interval between PS and first egg was calculated for each bird, as well as the weight of the first egg (if it had a normal shell and a single yolk). Body weight and external carcass morphology measurements were recorded at PS for each bird and again at sexual maturity. External measurements included right shank length, keel length, chest width, and thoracic girth. Right shank (tibiotarsus) length was measured with a digital caliper from the top of the hock joint to the footpad. Keel length was the distance from the hypocleidoclavical joint to the caudal end of the sternum (Griffin et al., 2005). Chest width was measured with digital calipers placed under the wings, 2.5 cm below the clavicle bones at the widest point on the chest. Chest girth during exhalation was measured with a fabric tape at the widest point on the breast, positioned snugly under the wings.
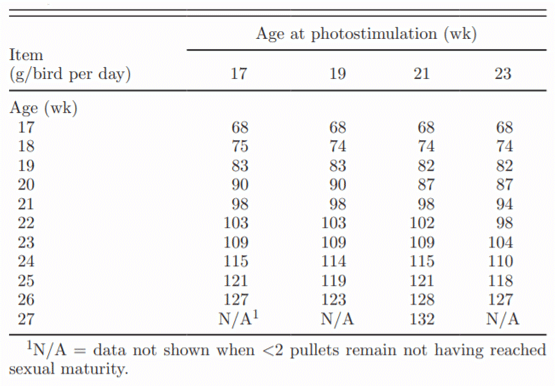
Table 1. Feed allocation from photostimulation to sexual maturity
On the day following its first egg, each bird was euthanized. Breast muscle weight was calculated as the sum of the weights of the pectoralis major and minor. The liver and abdominal fat pad were dissected and weighed. The fat pad included the fat adhering to the gizzard, but not the proventriculus. Oviduct weight (emptied of contents) was recorded. The ovary was weighed, and ovarian follicles greater than 10 mm in diameter were classified as large yellow follicles (LYF), and each LYF was individually weighed. The number of small yellow follicles (SYF; 5–10 mm diameter) and large white follicles (LWF; 2 to 5 mm diameter) were recorded. The incidence of atretic LYF and SYF, characterized by a discolored or shrunken appearance, was also noted (Renema et al., 1999). Stroma weight was the weight of the ovary after the LYF and atretic LYF had been removed. At 31 wk of age the study ended. Any remaining birds were killed, dissected, and reproductive status was confirmed.
Statistical Analyses
Growth and reproductive development data were analyzed by 2-way ANOVA with the MIXED procedure of SAS (SAS Institute Inc., 2002). Body weight at sexual maturity was analyzed by 2-way analysis of covariance (ANCOVA), with age at sexual maturity as a covariate. Carcass conformation data were analyzed by 2-way ANCOVA, with BW as a covariate. With the exception of measures of variability, PS treatment, feeding treatment, and their interactions were tested against the experimental unit of bird (PS × feeding program). Standard deviation in age at first egg was calculated at the environment chamber level and compared by ANOVA. These chambers have highly comparable environments due to the quality of environmental controls. Pair-wise differences between means were determined with the PDIFF option of the LSMEANS statement of SAS (SAS Institute Inc., 2002). Regression analyses were conducted with the REG procedure of SAS (SAS Institute Inc., 2002). Unless otherwise stated, all statements of significance were based upon P ≤ 0.05.
RESULTS AND DISCUSSION
Onset of Sexual Maturity
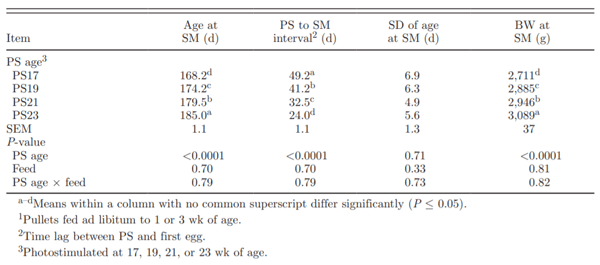
The initial duration of full feed access treatments did not affect age at first egg (data not shown). Concurring with previous research, birds that had a later PS age reached sexual maturity later (Table 2). Mean age at sexual maturity ranged from 168 d in the PS17 treatment to 185 d in the PS23 treatment. Photostimulating birds later reduced the interval between PS and sexual maturity from a high of 49.2 d in PS17 birds to a low of 24.0 d in PS23 pullets. For every day that PS was delayed, sexual maturity was delayed by only 0.40 d (R2 = 0.52, P < 0.0001). This supports previous hypotheses that the rate of sexual maturation increases when PS occurred later (Yuan et al., 1994; Robinson et al., 1996). Renema et al. (2001b) observed a similar increase in maturation rate of 0.35 d per d that PS was delayed. In other studies, the delay in maturation was even less. Lewis (2006) calculated an expected delay of 0.36 d per d that PS was delayed. Delayed maturation rates of 0.22, 0.27, and 0.21 d per day PS was delayed were observed by Robinson et al. (1996), Joseph et al. (2002a), and Ciacciariello and Gous (2005b), respectively. A total of 3 pullets did not reach sexual maturity by the end of the study: 2 from the PS17 group and 1 from the PS19 group.
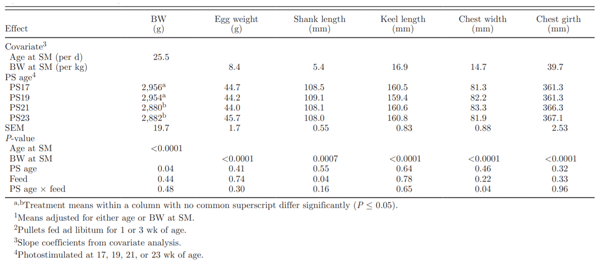
Table 2. Effect of photostimulation (PS) age and initial full feeding1on sexual maturity (SM) parameters
In the current study, birds weighed more at PS and sexual maturity, and had less abdominal fat pad at sexual maturity than what was reported by Robinson et al. (1996). Whereas the photosensitivity of breeding stock used in these trials may differ (Robinson et al., 1996) and body composition of the bird has been changing, rate of feeding during this period could also affect rate of maturation. Joseph et al. (2002a) fed birds 10 g/d (13.1%) more feed than in our experiment between 18 and 20 wk. Once the 70 kcal/kg lower ME content in their diet was accounted for, Joseph et al. (2002a) still fed 10.6% more ME during this period than in the current study. The feeding program between 15 to 20 wk can affect early egg production and feeding level may be even more important than PS age in affecting the sexual maturation process (Ciacciariello and Gous, 2005a). Renema et al. (2007b) further reported that recent feeding level can override BW effects. It must be recognized that though BW can be a good general predictor of sexual maturation, it is still a contextual measurement and can be modulated by feed intake. The general effects of BW were assessed by Lewis et al. (2007), who examined the effect of BW targets ranging from 1.91 to 2.74 kg at 20 wk of age on age at sexual maturity. Those authors reported that the acceleratory effect of increasing BW targets reached a maximum in the 2.54-kg group, with no additional effect of using a 2.74-kg target.
Neither feeding program (data not shown) nor age at PS (Table 2) affected the variation in age at sexual maturity, indicating that, contrary to expectations, later PS ages did not affect the uniformity of sexual maturity age. Renema et al. (2001a) reported that the CV of age at sexual maturity for birds PS at 19 or 21 wk was similar (5.02 or 4.02, respectively); this was in contrast with Robinson et al. (1996), who reported that one advantage of delaying PS was that the birds reached sexual maturity more uniformly. More recently, Renema et al. (2007b) reported a higher uniformity of entry into lay by PS at 22 compared with 18 wk of age. With later PS age, more birds would be approaching or in a mature physical state, reducing the diversion of nutrients away from support of the reproductive processes. As the relationship between PS age and the uniformity of sexual maturation age varied across experiments, more than PS age was likely contributing to these results. Increasing uniformity of sexual maturation has also been attempted by holding birds on a skip-a-day feeding pro-gram past PS rather than switching to an everyday feeding program (Gibson et al., 2008). This approach did not affect uniformity in the onset of lay, and the use of the skip-a-day treatment until 8% production resulted in reduced total egg production (155 vs. 177 eggs in total).
BW and Egg Weight at Sexual Maturity
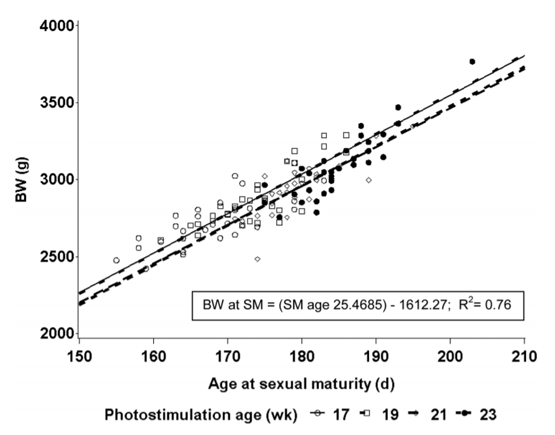
Birds of all treatments were on the same target BW profile, explaining why birds were heavier in later-maturing treatments (Table 2). On average, growth rate between PS and sexual maturity in each group was approximately 25.5 g/d (Table 3), which was consistent with previous studies (Lewis et al., 1997; Renema et al., 2007b). However, age itself did not fully explain the differences in BW. Covariate analysis of BW at first egg (corrected for age at sexual maturity) demonstrated that BW at sexual maturity was also affected by PS age (Table 3). Pullets PS at 17 and 19 wk of age were heavier at sexual maturity compared with those PS at 21 or 23 wk of age (Figure 1). This result may be due to an age-related shift in energy partitioning priorities away from BW gain to reproductive development. Pullets PS later were found to partition a greater proportion of their nutrient intake into reproductive organ development rather than into breast muscle. At later PS ages, a higher proportion of the flock was able to respond to the PS cue and commence reproductive development.
The proportion of carcass fat at sexual maturity increased, whereas the proportion of carcass protein was lower when later PS ages were used (Renema et al., 2007b). The later-PS birds had stored more nutrients before PS, diverting more nutrients toward support of reproductive development. In the current study, changing PS from 17 to 23 wk of age could therefore have allowed a larger partitioning of nutrient intake into fat deposition and reproductive organ development. Broiler genetic selection programs have resulted in broiler breeder hens that store less fat than in previous generations (Renema et al., 2007a). Methods to increase fat deposition in broiler breeder hens back to previous levels may contribute to increased tolerance of the hen to day-to-day changes in feed availability. Whereas the hen uses both fat and protein as energy sources, fat is not a metabolically active energy store. It is not clear at what point unintended negative consequences of low carcass fat on support of reproductive development will begin to occur.
The PS23 hens had heavier initial eggs (47.3 g) than PS17, PS19, or PS21 (mean = 43.8 g). This result was consistent with the general understanding that light hens produced lighter eggs and heavy hens produced heavier eggs (McDaniel et al., 1981). In the current study, covariate analysis adjusting for BW at sexual maturity demonstrated that egg weight differences were explained only by the increased BW at maturity in birds PS later and were otherwise not affected by PS treatment (Table 3). Even though egg size was positively correlated with hen size (McDaniel et al., 1981), this relationship can be modulated by feeding level around time of sexual maturity, with egg size increasing with higher feeding level (Zuidhof et al., 2007). Under more standard feeding conditions, differences in initial egg weight can be permanent. Joseph et al. (2002b) reported that hens PS at 23 wk of age laid larger eggs throughout the entire production cycle compared with those PS earlier, indicating that this was not a transient effect near to onset of lay.
Table 3. Covariate analysis1of carcass characteristics and egg weight at sexual maturity (SM) in response to photostimulation (PS)age and initial full feeding2
Carcass and Ovary Characteristics at Sexual Maturity
Covariate analysis demonstrated that differences in carcass parameters at sexual maturity were related to BW at sexual maturity (Table 3). Age at PS did not affect BW-corrected shank length at sexual maturity, suggesting that previous reports of increased frame size, fatness, and proportion of breast with use of later PS ages (Renema et al., 2001a, 2007b) can simply be explained by BW differences, with no additional effect of the later PS age itself. In the current study, when frame size data was not subject to covariate adjustment for BW, birds in the PS23 treatment had a larger frame size at first egg (6.4 mm longer keel length, 6.5 mm wider chest, and 19.6 mm greater thoracic girth) com-pared with PS17 pullets (data not presented). Frame size measures have been shown to be closely tied to BW (de Beer and Coon, 2007; Renema et al., 2007b). However, an additional PS age effect was hypothesized for this study, where pullets would be closer to their mature BW at later PS ages, thereby making more nutrients available for storage in the form of muscle or fat. However, later PS ages did not lead to a higher proportion of fat and muscle beyond what could be explained by simple BW differences.
Figure 1. Effect of age at sexual maturity (SM; d) on BW at first egg in pullets photostimulated at 17, 19, 21, or 23 wk of age.
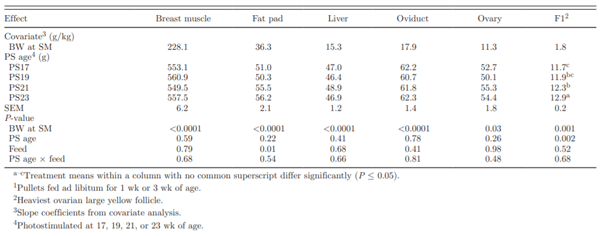
Table 4. Covariate analysis of effect of photostimulation (PS) age and initial full feeding1on abdominal fat pad, liver, and reproductive organ weights at sexual maturity (SM)
A carry-over effect of the early feeding treatments birds in the current study received before this experiment was a reduced thoracic width at sexual maturity in birds PS at 17 wk of age following AL1 as com-pared with AL3 (data not presented). Pishnamazi et al. (2008) demonstrated that, apart from BW uniformity and fatness, the effects of a 1- or 3-wk initial ad libitum feeding program were not detectable by 16 wk of age, so the minimal effect of early feeding on frame size in the current study was not surprising.
Non-BW-adjusted breast muscle weight was higher for PS23 birds than other PS treatments. Birds with a larger breast muscle weight in later PS treatments also had a larger frame size. Similar results were observed for the abdominal fat pad, liver, and reproductive organ weights. Brody et al. (1984) reported that birds PS at an older age had a larger fat pad. With a later PS, more pullets had a chance to approach their mature body size and transition to allocating more nutrients to tissue accretion. During puberty, the 2 major depots for lipids synthesized by the liver have been identified as the abdominal fat pad and the ovary, and it can be expected that the weights of these tissues should reflect the increased weight in liver due in part to increased lipid synthesis. However, once data were BW corrected, no differences in breast muscle weights, fat pad, liver, oviduct, or ovary weights were observed (Table 4).
Previous research has shown that abdominal fat pad weight as a percentage of BW was positively correlated with BW (r = 0.312), indicating that heavier birds had a greater proportion of fat (Renema et al., 2001a). The current study demonstrated that reported effects of PS age on carcass conformation traits (Renema et al., 2001a) and on reproductive morphology (Hocking, 2004) were likely simply linked to BW at sexual maturity.
At sexual maturity, the number of ovarian LYF and SYF were similar in all of the PS groups (Table 5). The number of LWF at sexual maturity increased with PS age (Table 5). The F1 ovarian LYF weight was increased by 0.18 g for every 100 g increase in BW at sexual maturity (r2 = 0.30; P = 0.001). Covariate analysis demonstrated that the F1 follicle weight was affected by PS age. Photostimulating progressively increased F1 weight by a total of 10.3% between the PS17 and PS23 treatments (Table 4). Plasma estrogen concentration in birds has previously been shown to increase at a faster rate in birds PS at 21 wk of age than in those PS at 19 wk of age (Renema et al., 2001a). The higher state of physical maturity before PS resulted in a greater physiological response to PS. During sexual maturation, sensitivity to estrogen has been reported to be high, and surplus nutrients were partitioned preferentially to the ovary under the influence of estrogen (Renema et al., 1999).
The incidence of atresia in ovarian LYF at sexual maturity was low (Table 5). However, both age at PS and feeding program affected the incidence of SYF atresia at sexual maturity. Atresia of SYF was higher in the PS17 and PS19 treatments compared with the PS21 and PS23 treatments (Table 5). Rates of SYF atresia has been reported to increase in birds with a longer sexual maturation time (Renema et al., 1999). It was not clear if this was an age-related phenomenon or a consequence of the longer PS to sexual maturity interval in birds PS earlier.
Table 5. Effect of photostimulation (PS) age and initial full feeding1on ovarian follicle characteristics at sexual maturity
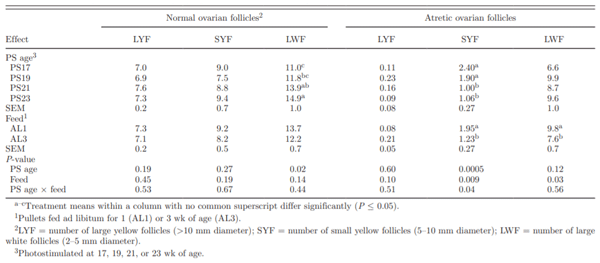
Allowing pullets an initial 3 wk of full access to feed (AL3) resulted in a lower incidence of SYF atresia compared with AL1 pullets (Table 5). Small but significant differences were noted in the incidence of SYF atresia in the interaction of feeding program and age at PS. The highest incidence of atresia was observed in the AL1 birds that were PS earlier (17 or 19 wk). Nutrient allocation programs that limit BW gain during sexual maturation resulted in an increased incidence of SYF atresia (Renema et al., 2007b). Whereas overall feed intake of AL1 and AL3 birds was similar for the 1- to 16-wk period, feed allocation for AL3 pullets between 12 to 16 wk of age was held constant due to their greater energetic efficiency, whereas AL1 birds received steady increases in feed allocation (Pishnamazi et al., 2008). Feed intake differences during this period have the potential to affect ovary development. As ovulation rate represented the balance of atresia and follicular recruitment (Renema et al., 2001a), the increased rates of atresia observed in earlier-maturing birds in the current study could have led to reduced egg production. Feed restriction appears to modify the development of reproductive hormone control, with the 6- to 15-wk period reported to be a critical rearing period for reproduction due to its effect on subsequent egg production (Brugge-man et al., 2005), and 16 wk of age being a key point for reproductive development in the broiler breeder female (Bruggeman et al., 1998). Feeding pullets to 55% of ad libitum compared with restricting them to the normal, smaller size was reported to increase overall egg production through an increased persistency of lay (Bruggeman et al., 2005).
In the current experiment, delaying PS from 17 to 23 wk of age only delayed sexual maturity by 17 d. Use of later PS ages caused pullets to mature faster and commence egg production at heavier BW, but had no effect on uniformity in age at first egg. Heavier egg, breast muscle, and abdominal fat pad weights found in birds that matured later were found to be related to heavier BW at sexual maturity rather than to PS age treatment. Use of later PS ages could be valuable in markets where large early egg size was important. A later PS increased LWF number and size of the largest preovulatory follicle (F1), independent of BW.
The current experiment was conducted in part to reevaluate the response of individually housed broiler breeders to a range of PS ages. Despite 10 yr of selection for growth traits, the interval between PS and sexual maturation has not changed since the study by Robinson et al. (1996). Whereas competition in a group-housed setting would add variability in feed intake, mean responses would still be expected to be similar to those of the current study. Carcass composition has shifted this interval toward a slightly less fat bird carrying a substantially higher proportion of breast muscle. However, in contrast to previously published work concerning PS age, differences in carcass and reproductive morphology due to PS age treatment were found to simply be BW effects. The relationship between BW and maturation age was very predictable (Figure 1). The results of the current experiment suggest that previously published research describing higher rates of egg production in flocks PS later may have been due in part to a more uniform onset of lay. The uniformity of individual ages of sexual maturation was similar among PS age groups ranging from 17 to 23 wk of age. This suggests that PS ages can be selected based on flock weight, with little concern about potential for lost egg production due to uniformity of response to PS.
ACKNOWLEDGMENTS
This study was supported by funds provided by the Alberta Agricultural Research Institute (Edmonton, Alberta, Canada), the Canadian Poultry Research Centre (Ottawa, Ontario), and Aviagen North America (Huntsville, AL). Special thanks to Lilydale Hatchery (Edmonton, Alberta, Canada) for their co-operation. Thanks to F. Dennis and the staff and students of the University of Alberta Poultry Research Centre for technical expertise.
This article was originally published in 2014 Poultry Science 93:1274–1281. http://dx.doi.org/ 10.3382/ps.2012-02834. This is an Open Access article under a Creative Commons License. 













.jpg&w=3840&q=75)