Chronic Stress, Chronic Inflammation, and Mitochondrial Dysfunction: The Silent Killers
Author details:
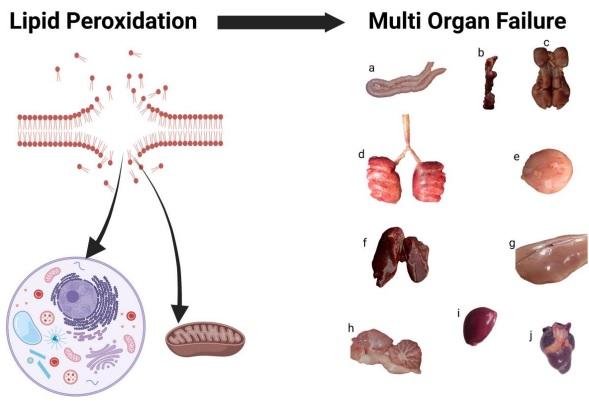
The gastrointestinal tract provides the biological environment for nutrient digestion and absorption. Its physical and chemical barriers are crucial to protecting from invading pathogens and toxic substances. On this basis, the intactness of the gastrointestinal tract with its multiple functions and impacts is one of the key prerequisites for human and animal health. There is no doubt that the functions of a healthy gut system also largely benefit the welfare and performance of animals in farming systems such as poultry industries. As a result of intensive genetic programs, broiler chickens grow rapidly due to the high absorption capacity of intestinal epithelia for nutrients, the quick transport of nutrients to the muscle, and their efficient conversion into energy and biomass. Reactive oxygen and nitrogen species are physiologically created by intestinal epithelial cells, either due to oxygen metabolism or enteric commensal bacteria, which are important in regulating gut health. On the other hand, increased generation of these oxidants goes along with the formation of free radicals resulting in oxidative stress causing lipid peroxidation and dramatic molecular changes in the structure and function of the cell and mitochondrial membranes. These effects contribute to chronic oxidative stress and inflammation of the gastrointestinal tract and generally affect all chicken organs, tissues, and cells. Hence, all forms of chronic stress, regardless of their origin, negatively impact the chickens' overall performance, health, and welfare. This review article highlights some enteric inflammation models and biomarkers to evaluate gut integrity in chickens and discusses the repercussions that chronic stress and intestinal inflammation have on the health and performance of commercial poultry.
Keywords: ceca, chickens, inflammation, oxidative stress, stress.
Aggrey, S.E., Karnuah, A.B., Sebastian, B., Anthony, N.B., 2010. Genetic properties of feed efficiency parameters in meat-type chickens. Genetics Selection Evolution, 42, pp.1-5. 10.1186/1297-9686-42-25.
Alverdy, J., Zaborina, O., Wu, L., 2005. The impact of stress and nutrition on bacterial-host interactions at the intestinal epithelial surface. Current Opinion in Clinical Nutrition and Metabolic Care 8, 205–209. 10.1097/00075197-200503000-00016.
Audy, J., Mathieu, O., Belvis, J., Tompkins, T., 2012. Transcriptomic response of immune signalling pathways in intestinal epithelial cells exposed to lipopolysaccharides, Gram-negative bacteria, or potentially probiotic microbes. Beneficial Microbes, 3, 273–286. 10.3920/BM2012.0027.
Awad, W.A., Hess, C., Hess, M., 2017. Enteric pathogens and their toxin-induced disruption of the intestinal barrier through alteration of tight junctions in chickens. Toxins, 9, 60. 10.3390/toxins9020060.
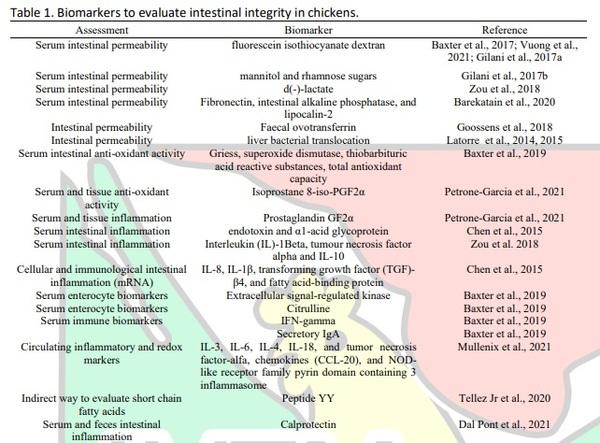
Barekatain, R., Howarth, G.S., Willson, N.L., Cadogan, D., Wilkinson, S., 2020. Excreta biomarkers in response to different gut barrier dysfunction models and probiotic supplementation in broiler chickens. Plos One, 15, e0237505. 10.1371/journal.pone.0237505.
Barros, T.L., Beer, L.C., Tellez, G., Fuller, A.L., Hargis, B.M., Vuong, C.N., 2020. Research Note: Evaluation of dietary administration of sodium chlorate and sodium nitrate for Histomonas meleagridis prophylaxis in turkeys. Poultry Science, 99, 1983-1987. 10.1016/j.psj.2019.11.055.
Bar-Shira, E., Sklan, D., Friedman, A., 2003. Establishment of immune competence in the avian GALT during the immediate post-hatch period. Developmental and Comparative Immunology, 27, 147–157. 10.1016/s0145-305x(02)00076-9.
Baxter, M.F., Merino-Guzman, R., Latorre, J.D., Mahaffey, B.D., Yang, Y., Teague, K.D., et al., 2017. Optimizing fluorescein isothiocyanate dextran measurement as a biomarker in a 24-h feed restriction model to induce gut permeability in broiler chickens. Frontiers in Veterinary Science, 4, 56. 10.3389/fvets.2017.00056.
Baxter, M.F., Latorre, J.D., Dridi, S., Merino-Guzman, R., Hernandez-Velasco, X., Hargis, B.M., et al., 2019. Identification of serum biomarkers for intestinal integrity in a broiler chicken malabsorption model. Frontiers in Veterinary Science, 6, 144. 10.3389/fvets.2019.00144.
Baxter, M.F., Greene, E.S., Kidd, M.T., Tellez-Isaias, G., Orlowski, S., Dridi, S., 2020. Water amino acid-chelated trace mineral supplementation decreases circulating and intestinal HSP70 and proinflammatory cytokine gene expression in heat-stressed broiler chickens. Journal of Animal Science, 98, skaa049. 10.1093/jas/skaa049.
Beer, L.C., Latorre, J.D., Rochell, S.J., Sun, X., Tellez, G., Fuller, A.L., et al., 2020. Research Note: Evaluation of deoxycholic acid for antihistomonal activity. Poultry Science, 99, 3481-3486. 10.1016/j.psj.2020.03.04.
Beer, L.C., Graham, B.D.M., Barros, T.L., Latorre, J.D., Tellez-Isaias, G., Fuller, A.L., et al., 2022. Evaluation of live-attenuated Histomonas meleagridis isolates as vaccine candidates against wild-type challenge. Poultry Science, 101, 101656. 10.1016/j.psj.2021.101656.
Bedford, M., Classen, H., 1993. An in vitro assay for prediction of broiler intestinal viscosity and growth when fed rye-based diets in the presence of exogenous enzymes. Poultry Science, 72, 137–143. 10.3382/ps.0720137.
Bedford, M., Schulze, H., 1998. Exogenous enzymes for pigs and poultry. Nutrition Research Reviews, 11, 91–114. 10.1079/NRR19980007.
Berger, M., Gray, J.A., Roth, B.L., 2009. The expanded biology of serotonin. Annual Review of Medicine, 60, 355–366. 10.1146/annurev.med.60.042307.110802.
Bickler, S.W., Prieto, J.M., Cauvi, D.M., De Cos, V., Nasamran, C., Ameh, E., et al., 2020. Differential expression of nuclear genes encoding mitochondrial proteins from urban and rural populations in Morocco. Cell Stress Chaperones, 25, 847-856. 10.1007/s12192-020-01108-x.
Binsiya, T., Sejian, V., Bagath, M., Krishnan, G., Hyder, I., Manimaran, A., Lees, A., Gaughan, J.,.Bhatta, R., 2017. Significance of hypothalamic-pituitary[1]adrenal axis to adapt to climate change in livestock. International Research Journal of Agricultural and Food Sciences, 2, 1–20. http://www.prudentjournals.org/IRJAFS.
Biswal, J., Vijayalakshmy, K., Bhattacharya, T.K, Rahman, H., 2021. Impact of heat stress on poultry production. World's Poultry Science Journal, 78, 179-196. 10.1080/00439339.2022.2003168.
Blake, D.P., Clark, E.L., Macdonald, S.E., Thenmozhi, V., Kundu, K., Garg, R., et al., 2015. Population, genetic, and antigenic diversity of the apicomplexan Eimeria tenella and their relevance to vaccine development. Proceedings of the National Academy of Science of the United States of America, 112, E5343-E5350. 10.1073/pnas.1506468112
Blake, D.P., Knox, J., Dehaeck, B., Huntington, B., Rathinam, T., Ravipati, V., et al., 2020. Re-calculating the cost of coccidiosis in chickens. Veterinary Research, 51, 115. 10.1186/s13567-020-00837-2.
Bloom, S.R., 1987. Gut hormones in adaptation. Gut, 28, 31–35. 10.1136/gut.28.suppl.31.
Braun, E.J., 1999. Integration of renal and gastrointestinal function. The Journal of Experimental Zoology, 283, 495–499.
Calefi, A., W. Quinteiro-Filho, A. Ferreira, Palermo-Neto, J., 2017. Neuroimmunomodulation and heat stress in poultry. World’s Poultry Science Journal, 73, 493–504. 10.1017/S0043933917000472.
Castanon, J. 2007. History of the use of antibiotic as growth promoters in European poultry feeds. Poultry Science, 86, 2466–2471. https://doi.org/10.3382/ps.2007-00249.
Casteleyn, C., Doom, M., Lambrechts, E., Van Den Broeck, W., Simoens, P., Cornillie, P., 2010. Locations of gut-associated lymphoid tissue in the 3-month-old chicken: a review. Avian Pathology, 39, 143–150. 10.1080/03079451003786105.
Cao, C., Chowdhury, V.S., Cline, M.A., Gilbert, E.R., 2021. The Microbiota-Gut-Brain Axis During Heat Stress in Chickens: A Review. Frontiers in Physiology, 12, 752265. 10.3389/fphys.2021.752265.
Celluzzi, A., Masotti, A., 2016. How our other genome controls our epi-genome. Trends in Microbiology, 24, 777–787. 10.1016/j.tim.2016.05.005.
Chapman, H.D., 2008. Coccidiosis in the turkey. Avian Pathology, 37, 205–223. 10.1080/03079450802050689.
Chapman, H.D., 2014. Milestones in avian coccidiosis research: a review. Poultry Science, 93, 501–511. 10.3382/ps.2013-03634.
Chen, J., Tellez, G., Richards, J.D., Escobar, J., 2015. Identification of potential biomarkers for gut barrier failure in broiler chickens. Frontiers in Veterinary Science, 2, 14. 10.3389/fvets.2015.00014.
Chen, Y., Kevil, C.G., 2020. Redox signaling and cardiovascular disease: new paradigms and discoveries. Redox Biology, 37, 101743. doi: 10.1016/j.redox.2020.101743.
Chen, J., Yan, F., Kuttappan, V.A., Wedekind, K., Vázquez-Añón, M., Hancock, D., 2023. Effects of bis-chelated copper in growth performance and gut health in broiler chickens subject to coccidiosis vaccination or coccidia challenge. Frontiers in Physiology, 13, 2775. 10.3389/fphys.2022.991318.
Choct, M., Hughes, R.J., Trimble, R.P., Angkanaporn, K., Annison, G., 1995. Non-starch polysaccharide-degrading enzymes increase the performance of broiler chickens fed wheat of low apparent metabolizable energy. The Journal of Nutrition, 125, 485–492. 10.1093/jn/125.3.485.
Clark, E.L., Tomley, F.M., Blake, D.P., 2017. Are Eimeria genetically diverse, and does it matter? Trends in Parasitology, 33, 231-241. 10.1016/j.pt.2016.08.007.
Cobb-Vantress., 2022. Cobb500 Broiler Performance & Nutrition Supplement. https://www.cobb-vantress.com/assets/Cobb-Files/product[1]guides/5502e86566/2022-Cobb500-Broiler-Performance-Nutrition-Supplement.pdf [Accessed January 20, 2022].
Cohen, H., Zohar, J., Gidron, Y., Matar, M.A., Belkind, D., Loewenthal, U., Kozlovsky, N., Kaplan, Z., 2006. Blunted HPA axis response to stress influences susceptibility to posttraumatic stress response in rats. Biological Psychiatry, 59, 1208-1218. 10.1016/j.biopsych.2005.12.003.
Coles, M.E., Forga, A.J., Señas-Cuesta, R., Graham, B.D., Selby, C.M., Uribe, A.J., et al., 2021. Assessment of Lippia origanoides essential oils in a Salmonella Typhimurium, Eimeria maxima, and Clostridium perfringens challenge model to induce necrotic enteritis in broiler chickens. Animals, 11, 1111. 10.3390/ani11041111.
Collett, S.R., 2012. Nutrition and wet litter problems in poultry. Animal Feed Science and Technology, 173, 65-75. 10.1016/j.anifeedsci.2011.12.013.
Collins, S., Pi, J., Yehuda-Shnaidman, E., 2012. Uncoupling and reactive oxygen species (ROS)-a double-edged sword for beta-cell function “Moderation in all things.” Best Practice & Research Clinical Endocrinology and Metabolism, 26, 753–758. 10.1016/j.beem.2012.08.002.
Collins, J.W. and Siegel, P.B., 1987. Human handling, flock size and responses to an E. coli challenge in young chickens. Applied Animal Behaviour Science, 19(1-2), pp.183-188.
Cryan, J.F., Dinan, T.G., 2012. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nature Reviews Neuroscience, 13, 701–712. 10.1038/nrn3346.
Dahiya, J., Wilkie, D., Van Kessel, A., Drew, M., 2006. Potential strategies for controlling necrotic enteritis in broiler chickens in post-antibiotic era. Animal Feed Science and Technology, 129, 60–88. 10.1016/j.anifeedsci.2005.12.003.
Dal Pont, G.C., Farnell, M., Farnell, Y., Kogut, M.H., 2020. Dietary factors as triggers of low-grade chronic intestinal inflammation in poultry. Microorganisms, 8, 139. 10.3390/microorganisms8010139.
Dal Pont, G.C., Belote, C.B.L., Lee, A., Bortoluzzi, C., Eyng, C., Sevastiyanova, M., et al., 2021. Novel models for chronic intestinal inflammation in chickens: intestinal inflammation pattern and biomarkers. Frontiers in Immunology, 12, 676628. 10.3389/fimmu.2021.676628.
Debon, S. J., Tester, R.F., 2001. In vitro binding of calcium, iron and zinc by non-starch polysaccharides. Food Chemistry, 73, 401–410. 10.1016/S0308-8146(00)00312-5.
Desvaux, M., Hébraud, M., Talon, R., Henderson I.R., 2009. Secretion and subcellular localizations of bacterial proteins: a semantic awareness issue. Trends in Microbiology, 17, 139–145. 10.1016/j.tim.2009.01.004.
Diaz Carrasco, J.M., Casanova, N.A. and Fernández Miyakawa, M.E., 2019. Microbiota, gut health and chicken productivity: what is the connection?. Microorganisms, 7(10), p.374.
Dibner, J., Atwell, C., Kitchell, M., Shermer, W., Ivey, F., 1996. Feeding of oxidized fats to broilers and swine: effects on enterocyte turnover, hepatocyte proliferation and the gut associated lymphoid tissue. Animal feed Science and Technology, 62, 1–13. 10.1016/S0377-8401(96)01000-0.
Dimitrov, D.V., 2011. The human gutome: Nutrigenomics of the host-microbiome interactions. OMICS, 15, 419–430. 10.1089/omi.2010.0109.
Duke, G.E., 1982. Gastrointestinal motility and its regulation. Poultry Science, 61, 1245–1256. 10.3382/ps.0611245.
Duke, G., Evanson, O., Epstein, D., 1983. Coordination of cecal motility during cecal evacuation. Poultry Science, 62:545–550. 10.3382/ps.0620545.
Duke, G.E., 1999. Mechanisms of excreta formation and elimination in turkeys and ostriches. The Journal of Experimental Zoology, 283, 478–479. 10.1002/(sici)1097-010x(19990301/01)283:4/5<478::aid-jez18>3.0.co;2-e
Dunaway, A., Adedokun, S.A., 2021. Coccidia vaccine challenge and exogenous enzyme supplementation in broiler chicken 1. Effect on digesta viscosity, diet energy utilization, and apparent metabolizable energy value of wheat. Animals, 11, 641. 10.3390/ani11030641.
Edelblum, K. L., Turner. J.R., 2009. The tight junction in inflammatory disease: communication breakdown. Current Opinion in Pharmacology, 9, 715–720. 10.1016/j.coph.2009.06.022
Elson, C.O., Cong, Y., 2012. Host-microbiota interactions in inflammatory bowel disease. Gut Microbes, 3, 332–344. 10.4161/gmic.20228.
Fasano, A., 2011. Zonulin and its regulation of intestinal barrier function: the biological door to inflammation, autoimmunity, and cancer. Physiological Review, 91, 151–175. 10.1152/physrev.00003.2008.
Fasano, A., 2020. All disease begins in the (leaky) gut: Role of zonulin-mediated gut permeability in the pathogenesis of some chronic inflammatory diseases. F1000Res, 9, F1000. 10.12688/f1000research.20510.1.
Feye, K.M., Baxter, M.F.A., Tellez-Isaias, G., Kogut, M.H. and Ricke, S.C., 2020. Influential factors on the composition of the conventionally raised broiler gastrointestinal microbiomes. Poultry science, 99(2), pp.653-659.
Federico, A., Morgillo, F., Tuccillo, C., Ciardiello, F., Loguercio, C., 2007. Chronic inflammation and oxidative stress in human carcinogenesis. International Journal of Cancer, 121, 2381-2386. 10.1002/ijc.23192.
Fernandez-Siurob, I., Retana, M.A., Tellez, G., Arroyo-Navarro, L., Bañuelos-Hernandez, B., Castellanos-Huerta, I., 2014. Assessment of viral interference using a chemical receptor blocker against avian influenza and establishment of protection levels in field outbreaks. Vaccine, 32, 1318-1322. 10.1016/j.vaccine.2014.01.006.
Feye, K., Baxter, M., Tellez-Isaias, G., Kogut, M., Ricke, S., 2020. Influential factors on the composition of the conventionally raised broiler gastrointestinal microbiomes. Poultry Science, 99, 653–659. 10.1016/j.psj.2019.12.013.
Forsythe, P., Sudo, N., Dinan, T., Taylor, V. H., Bienenstock, J., 2010. Mood and gut feelings. Brain, Behavior and Immunity, 24, 9–16. 10.1016/j.bbi.2009.05.058.
Francesch, M., Brufau, J., 2007. Nutritional factors affecting excreta/litter moisture and quality. World's Poultry Science Journal, 60, 64-75. 10.1079/WPS20035.
Frye, R.E., Rose, S., Chacko, J., Wynne, R., Bennuri, S.C., Slattery, J.C., et al., 2016. Modulation of mitochondrial function by the microbiome metabolite propionic acid in autism and control cell lines. Translational Psychiatry, 6, e927. 10.1038/tp.2016.189.
Fukui, H., Xu, X., Miwa, H., 2018. Role of gut microbiota-gut hormone axis in the pathophysiology of functional gastrointestinal disorders. Journal of Neurogastroenterology and Motility, 24, 367. 10.5056/jnm18071.
Gabriel, I., Lessire, M., Mallet, S., Guillot, J., 2006. Microflora of the digestive tract: critical factors and consequences for poultry. World’s Poultry Science Journal, 62, 499–511. 10.1017/S0043933906001115.
Gholamiandehkordi, A.R., Timbermont, L., Lanckriet, A., Broeck, W.V.D., Pedersen, K., Dewulf, J., Pasmans, F., Haesebrouck, F., Ducatelle, R., Immerseel, F.V., 2007. Quantification of gut lesions in a subclinical necrotic enteritis model. Avian Pathology, 36, 375–382. 10.1080/03079450701589118.
Gilani, S., Howarth, G.S., Kitessa, S.M., Tran, C.D., Forder, R.E.A., Hughes, R.J., 2017a. New biomarkers for increased intestinal permeability induced by dextran sodium sulphate and fasting in chickens. Journal of Animal Physiology and Animal Nutrition, 101, e237-e245. 10.1111/jpn.12596. 10.1111/jpn.12596.
Gilani, S., Howarth, G.S., Kitessa, S.M., Tran, C.D., Forder, R.E.A., Hughes, R.J., 2017b. Intestinal permeability induced by lipopolysaccharide and measured by lactulose, rhamnose and mannitol sugars in chickens. Animals, 11, 1174-1179. 10.1017/S1751731116002470.
Goldstein, D.L., 2006. Regulation of the avian kidney by arginine vasotocin. General and Comparative Endocrinology, 147, 78–84. 10.1016/j.ygcen.2005.09.018,
Goossens, E., Debyser, G., Callens, C., De Gussem, M., Dedeurwaerder, A., Devreese, B., et al., 2018. Elevated faecal ovotransferrin concentrations are indicative for intestinal barrier failure in broiler chickens. Veterinary Research, 49, 51. 10.1186/s13567-018-0548-4.
Gostner, J.M., Becker, K., Fuchs, D., Sucher, R., 2013. Redox regulation of the immune response. Redox Report Communications in Free Radical Research, 18, 88–94. 10.1179/1351000213Y.0000000044.
Gray, M.W., 2017. Lynn Margulis and the endosymbiont hypothesis: 50 years later. Molecular Biology of the Cell, 28, 1285–1287. 10.1091/mbc.E16-07-0509.
Gribble, F.M., Reimann, F., 2017. Signalling in the gut endocrine axis. Physiology & Behavior. 176, 183–188. 10.1016/j.physbeh.2017.02.039.
Gribble, F.M., Reimann, F., 2019. Function and mechanisms of enteroendocrine cells and gut hormones in metabolism. Nature Reviews Endocrinology, 15, 226–237. 10.1038/s41574-019-0168-8.
Griffiths, H.R., 2005. ROS as signaling molecules in T cells-evidence for abnormal redox signaling in the autoimmune disease, rheumatoid arthritis. Redox Report Communications in Free Radical Research, 10, 273–280. 10.1179/135100005X83680.
Guilliams, T.G., Edwards, L., 2010. Chronic stress and the hpa axis: clinical assessment and therapeutic considerations. A Review of Natural and Nutraceutical Therapies for Clinical Practice, 9, 1-12. www.pointinstitute.org/wp-content/uploads/2012/10/standard_v_9.2_hpa_axis.pdf
Hall, A.J., Duke, G.E., 2000. Effect of selective gastric intrinsic denervation on gastric motility in turkeys. Poultry Science, 79, 240–244. 10.1093/ps/79.2.240.
Heckert, R.A., Estevez, I., Russek-Cohen, E. and Pettit-Riley, R., 2002. Effects of density and perch availability on the immune status of broilers. Poultry Science, 81(4), pp.451-457.
Hentchel, K. L., Escalante-Semerena, J.C., 2015. Acylation of biomolecules in prokaryotes: a widespread strategy for the control of biological function and metabolic stress. Microbiology and Molecular Biology Reviews, 79, 321–346. 10.1128/MMBR.00020-15
Hernandez-Patlan, D., Solis-Cruz, B., Patrin Pontin, K., Latorre, J.D., Baxter, M.F., Hernandez-Velasco, X., et al., 2019. Evaluation of the dietary supplementation of a formulation containing ascorbic acid and a solid dispersion of curcumin with boric acid against Salmonella Enteritidis and necrotic enteritis in broiler chickens. Animals, 9, 184. 10.3390/ani9040184.
Hernández-Ramírez, J.O., Merino-Guzmán, R., Téllez-Isaías, G., Vázquez-Durán, A., Méndez-Albores A., 2021. Mitigation of AFB1-related toxic damage to the intestinal epithelium in broiler chickens consumed a yeast cell wall fraction. Frontiers in Veterinary Science, 8, 677965. 10.3389/fvets.2021.677965.
Hess, M., Liebhart, D., Bilic, I., Ganas P., 2015. Histomonas meleagridis—new insights into an old pathogen. Veterinary Parasitology, 208, 67–76. 10.1016/j.vetpar.2014.12.018.
Hu, Y.-J., Wang, Y.-D., Tan, F.-Q., Yang, W.X., 2013. Regulation of paracellular permeability: factors and mechanisms. Molecular Biology Reports, 40, 6123–6142.
Iebba, V., Totino, V., Gagliardi, A., Santangelo, F., Cacciotti, F., Trancassini, M. et al., 2016. Eubiosis and dysbiosis: the two sides of the microbiota. The New Microbiologica, 39, 1–12.
Ilan, Y., 2012. Leaky gut and the liver: a role for bacterial translocation in nonalcoholic steatohepatitis. World Journal of Gastroenterology, 18, 2609-2618. 10.3748/wjg.v18.i21.2609.
Itri, R., Junqueira, H.C., Mertins, O., Baptista, M.S., 2014. Membrane changes under oxidative stress: the impact of oxidized lipids. Biophysical Reviews, 6, 47–61. 10.1007/s12551-013-0128-9.
Jeon, M. K., Klaus, C., Kaemmerer, E., Gassler, N., 2013. Intestinal barrier: Molecular pathways and modifiers. World Journal of Gastrointestinal Pathophysiology, 4, 94. 10.4291/wjgp.v4.i4.94.
Jiang, Z., Schatzmayr, G., Mohnl, M., Applegate, T., 2010. Net effect of an acute phase response—Partial alleviation with probiotic supplementation. Poultry Science, 89, 28–33. 10.3382/ps.2009-00464.
Juruena, M.F., Bourne, M., Young, A.H., Cleare, A.J., 2021. Hypothalamic-pituitary-adrenal axis dysfunction by early life stress. Neuroscience Letters, 759, 136037. 10.1016/j.neulet.2021.136037.
Jones, D.P., Sies, H., 2015. The redox code. Antioxidants Redox Signal, 23, 734–746. 10.1089/ars.2015.6247.
Jonkers, D., Stockbrügger, R., 2003. Probiotics and inflammatory bowel disease. Journal of the Royal Society of Medicine, 96, 167–171.
Kallapura, G., Pumford, N.R., Hernandez-Velasco, X., Hargis, B.M., Tellez, G., 2014. Mechanisms involved in lipopolysaccharide derived ROS and RNS oxidative stress and septic shock. Journal of Microbiology Research and Reviews, 2, 6–11.
Karaca, Z., Grossman, A. and Kelestimur, F., 2021. Investigation of the Hypothalamo-pituitary-adrenal (HPA) axis: a contemporary synthesis. Reviews in Endocrine and Metabolic Disorders, 22, 179-204. 10.1007/s11154-020-09611-3.
Karasawa, Y., Duke, G.E., 1995. Effects of cecal ligation and colostomy on motility of the rectum, ileum, and cecum in turkeys. Poultry Science, 74, 2029–2034. 10.3382/ps.0742029.
Kelleher, B., Leahy, J., Henihan, A., O’dwyer, T., Sutton, T., Leahy, M., 2002. Advances in poultry litter disposal technology-a review. Bioresources Technology, 83, 27–36. 10.1016/s0960-8524(01)00133-x.
Khansari, N., Shakiba, Y., Mahmoudi, M., 2009. Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Patents on Inflammation & Allergy Drug Discovery, 3, 73–80. doi: 10.2174/187221309787158371
Kiarie, E., Romero, L.F., Nyachoti, C.M., 2013. The role of added feed enzymes in promoting gut health in swine and poultry. Nutrition Research Reviews, 26, 71–88. 10.1017/S0954422413000048.
Kim, W., Patterson, P., 2003. Effect of minerals on activity of microbial uricase to reduce ammonia volatilization in poultry manure. Poultry Science, 82, 223–231. 10.1093/ps/82.2.223.
Kim, Y. S., Ho, S.B., 2010. Intestinal goblet cells and mucins in health and disease: recent insights and progress. Current Gastroenterology Reports, 12, 319–330. 10.1007/s11894-010-0131-2
Kimball, R.T., Guido, M., Hosner, P.A. and Braun, E.L., 2021. When good mitochondria go bad: Cyto-nuclear discordance in landfowl (Aves: Galliformes). Gene, 801, 145841. 10.1016/j.gene.2021.145841.
Kogut, M.H., Genovese, K.J., Swaggerty, C.L., He, H., Broom, L., 2018. Inflammatory phenotypes in the intestine of poultry: not all inflammation is created equal. Poultry Science, 97, 2339–2346. 10.3382/ps/pey087.
Korniluk, A., Koper, O., Kemona, H., Dymicka-Piekarska, V., 2017. From inflammation to cancer. Irish Journal of Medical Science, 186, 57–62. 10.1007/s11845-016-1464-0.
Korver, D.R., 2012. Implications of changing immune function through nutrition in poultry. Animal Feed Science Technology, 173, 54-64. 10.1016/j.anifeedsci.2011.12.019.
Kuenzel, W., Jurkevich, A., 2010. Molecular neuroendocrine events during stress in poultry. Poultry Science, 89, 832–840. 10.3382/ps.2009-00376.
Kuttappan, V., Berghman, L., Vicuña, E., Latorre, J., Menconi, A., Wolchok, J., 2015. Poultry enteric inflammation model with dextran sodium sulfate mediated chemical induction and feed restriction in broilers. Poultry Science, 94, 1220-1226. 10.3382/ps/pev114
Lagler, J., Schmidt, S., Mitra, T., Stadler, M., Wernsdorf, P., Grafl, B., et al., 2021. Comparative investigation of IFN-γ-producing T cells in chickens and turkeys following vaccination and infection with the extracellular parasite Histomonas meleagridis. Developmental and Comparative Immunology, 116, 103949. 10.1016/j.dci.2020.103949.
Lane, N., 2005. Power, Sex, Suicide: Mitochondria and the Meaning of Life. (Oxford University Press., New York). Pp. 512.
Lara, L. J., Rostagno, M.H., 2013. Impact of heat stress on poultry production. Animals, 3, 356–369. 10.3390/ani3020356.
Latorre, J.D., Hernandez-Velasco, X., Kogut, M.H., Vicente, J.L., Wolfenden, R., Wolfenden, A., et al., 2014. Role of a Bacillus subtilis direct-fed microbial on digesta viscosity, bacterial translocation, and bone mineralization in turkey poults fed with a rye-based diet. Frontiers in Veterinary Science, 1, 26. 10.3389/fvets.2014.00026.
Latorre, J.D., Hernandez-Velasco, X., Bielke, L.R., Vicente, J.L., Wolfenden, R., Menconi, A., et al., 2015. Evaluation of a Bacillus direct-fed microbial candidate on digesta viscosity, bacterial translocation, microbiota composition and bone mineralisation in broiler chickens fed on a rye-based diet. British Poultry Science, 56, 723-732. 10.1080/00071668.2015.1101053.
Latorre, J. D., Hernandez-Velasco, X. Wolfenden, R. E. Vicente, J. L. Wolfenden, A. D. Menconi, A. et al., 2016. Evaluation and selection of Bacillus species based on enzyme production, antimicrobial activity, and biofilm synthesis as Direct-Fed microbial candidates for poultry. Frontiers in Veterinary Science, 3, 95. 10.3389/fvets.2016.00095.
Lauridsen, C., 2019. From oxidative stress to inflammation: redox balance and immune system. Poultry Science, 98, 4240–4246. 10.3382/ps/pey407.
Lazcano, A., Peretó, J., 2021. Prokaryotic symbiotic consortia and the origin of nucleated cells: A critical review of Lynn Margulis hypothesis. Biosystems, 204, 104408. 10.1016/j.biosystems.2021.10440.
Lee, M.Y., Griendling, K.K., 2008. Redox signaling, vascular function, and hypertension. Antioxidants & Redox Signaling, 10, 1045–1059. 10.1089/ars.2007.1986.
Leyva-Diaz, A.A., Hernandez-Patlan, D., Solis-Cruz, B., Adhikari, B., Kwon, Y.M., Latorre, J.D. et al., 2021. Evaluation of curcumin and copper acetate against Salmonella Typhimurium infection, intestinal permeability, and cecal microbiota composition in broiler chickens. Journal of Animal Science and Biotechnology, 12, 1–12. 10.1186/s40104-021-00545-7.
Li, J., Jia, H., Cai, X., Zhong, H., Feng, Q., Sunagawa, S., et al., 2014. An integrated catalog of reference genes in the human gut microbiome. Nature Biotechnology, 32, 834-841. 10.1038/nbt.2942.
Lian, P., Braber, S., Garssen, J., Wichers, H. J., Folkerts, G., Fink-Gremmels, J., Varasteh, S., 2020. Beyond heat stress: intestinal integrity disruption and mechanism-based intervention strategies. Nutrients, 12, 734. 10.3390/nu12030734.
Liang, X., Bushman, F.D., FitzGerald, G.A., 2014. Time in Motion: The molecular clock meets the microbiome. Cell, 159, 469–470. 10.1016/j.cell.2014.10.020.
Lichtenthaler, H.K., 1998. The stress concept in plants: an introduction. Annals of the New York Academy of Sciences, 851, 187–198. 10.1111/j.1749-6632.1998.tb08993.x.
Liebhart, D., Sulejmanovic, T., Grafl, B., Tichy, A., Hess, M., 2013. Vaccination against histomonosis prevents a drop in egg production in layers following challenge. Avian Pathology, 42, 79-84. 10.1080/03079457.2012.760841.
Liebhart, D., Ganas, P., Sulejmanovic, T., Hess, M., 2017. Histomonosis in poultry: previous and current strategies for prevention and therapy. Avian Pathology, 46 ,1–18. 10.1080/03079457.2016.1229458.
Lipton, B.H., 2016. The Biology of Belief: Unleashing the Power of Consciousness, Matter & Miracles. ( Hay House Inc., USA), Pp. 312.
Liversidge, A., 1993. Bacterial consciousness: Why spirochetes think as we do. Omni, 16, 10–11.
López-González, I., Aso, E., Carmona, M., Armand-Ugon, M., Blanco, R., Naudi, A.R., et al., 2015. Neuroinflammatory gene regulation, mitochondrial function, oxidative stress, and brain lipid modifications with disease progression in tau P301S transgenic mice as a model of frontotemporal lobar degeneration-tau. Journal of Neuropathology and Experimental Neurology, 74, 975–999. 10.1097/NEN.0000000000000241.
Lu, S., Wei, F., Li, G., 2021. The evolution of the concept of stress and the framework of the stress system. Cell Stress, 5,76. 10.15698/cst2021.06.250.
Lund, M.L., Egerod, K.L., Engelstoft, M.S., Dmytriyeva, O., Theodorsson, E., Patel, B.A., et al., 2018. Enterochromaffin 5-HT cells-A major target for GLP-1 and gut microbial metabolites. Molecular Metabolism, 11, 70–83. 10.1016/j.molmet.2018.03.00.
McAllister, M.M., 2014. Successful vaccines for naturally occurring protozoal diseases of animals should guide human vaccine research: A review of protozoal vaccines and their designs. Parasitology, 141, 624-640. 10.1017/S0031182013002060.
Maslowski, K.M., Mackay, C.R., 2010. Diet, gut microbiota and immune responses. Nature Immunology, 12, 5–9. 10.1038/ni0111-5.
Mayer, E.A., Knight, R., Mazmanian, S.K., Cryan, J.F., Tillisch, K., 2014. Gut microbes and the brain: paradigm shift in neuroscience. The Journal of Neuroscience, 34, 15490–15496. 10.1523/JNEUROSCI.3299-14.2014.
Megur, A., Baltriukiene, D., Bukelskiene, V., Burokas A., 2021. The microbiota-gut-brain axis and Alzheimer’s Disease: neuroinflammation is to blame? Nutrients, 13, 37. 10.1523/JNEUROSCI.3299-14.201.
Menconi, A., Hernandez-Velasco, X., Vicuña, E., Kuttappan, V., Faulkner, O., Tellez, G., et al., 2015. Histopathological and morphometric changes induced by a dextran sodium sulfate (DSS) model in broilers. Poultry Science, 94, 906-911. 10.3382/ps/pev054.
Mishra, B., Jha, R., 2019. Oxidative stress in the poultry gut: Potential challenges and interventions. Frontiers in Veterinary Science, 6, 60. 10.3389/fvets.2019.00060.
Mitra, T., Kidane, F.A., Hess, M., Liebhart, D., 2018. Unravelling the immunity of poultry against the extracellular protozoan parasite Histomonas meleagridis is a cornerstone for vaccine development: A review. Frontiers in Immunology, 9, 2518. 10.3389/fimmu.2018.02518.
Mitra, T., Bramberger, B., Bilic, I., Hess, M., Liebhart, D., 2021. Vaccination against the protozoan parasite Histomonas meleagridis primes the activation of toll-like receptors in turkeys and chickens determined by a set of newly developed multiplex RT-qPCRs. Vaccines, 9, 960. 10.3390/vaccines9090960.
Moore, P.A., Miles, D., Burns, R., Pote, D., Berg, K., Choi, IH., 2011. Ammonia emission factors from broiler litter in barns, in storage, and after land application. Journal of Environmental Quality, 40, 1395–1404. 10.2134/jeq2009.0383.
Morales-Barrera, E., Calhoun, N., Lobato-Tapia, J.L., Lucca, V., Prado-Rebolledo, O., Hernandez-Velasco, X., et al., 2016. Risks involved in the use of enrofloxacin for Salmonella Enteritidis or Salmonella Heidelberg in commercial poultry. Frontiers in Veterinary Science, 3 ,72. 10.3389/fvets.2016.00072.
Morales-Mena, A., Martínez-González, S., Teague, K.D., Graham, L.E., Señas-Cuesta, R., Vuong, C.N., et al., 2020. Assessment of fermented soybean meal on Salmonella Typhimurium infection in neonatal turkey poults. Animals, 10, 1849. 10.3390/ani1010184.
Mullenix, G.J., Greene, E.S., Emami, N.K., Tellez-Isaias, G., Bottje, W.G., Erf, G.F., et al., 2021. Spirulina Platensis inclusion reverses circulating pro[1]inflammatory (chemo) cytokine profiles in broilers fed low-protein diets. Frontiers in Veterinary Science, 8, 640968. 10.3389/fvets.2021.640968.
Naga Raja Kumari, K. and Narendra Nath, D., 2018. Ameliorative measures to counter heat stress in poultry. World's Poultry Science Journal, 74(1), pp.117-130.
Nahm, K., 2003. Evaluation of the nitrogen content in poultry manure. World’s Poultry Science Journal, 59, 77–88. 10.1079/WPS20030004.
Negri, S., Commisso, M., Avesani, L., Guzzo, F., 2021. The case of tryptamine and serotonin in plants: a mysterious precursor for an illustrious metabolite. Journal of Experimental Botany, 72, 5336–5355. 10.1093/jxb/erab220.
Neuman, H., Debelius, J.W., Knight, R., Koren, O., 2015. Microbial endocrinology: the interplay between the microbiota and the endocrine system. FEMS Microbiology Reviews, 39, 509-521. 10.1093/femsre/fuu010.
Nicol, C.J., Brown, S.N., Glen, E., Pope, S.J., Short, F.J., Warriss, P.D., Zimmerman, P.H. and Wilkins, L.J., 2006. Effects of stocking density, flock size and management on the welfare of laying hens in single-tier aviaries. British poultry science, 47(2), pp.135-146.
Niki, E., Yamamoto, Y., Komuro, E., Sato, K., 1991. Membrane damage due to lipid oxidation. The American Journal of Clinical Nutrition, 53, 201S–205S. 10.1093/ajcn/53.1.201S.
Noguchi, M., Hiwatashi, N., Liu, Z., Toyota, T., 1998. Secretion imbalance between tumour necrosis factor and its inhibitor in inflammatory bowel disease. Gut, 43, 203–209. 10.1136/gut.43.2.203.
Oakley, B.B., Lillehoj, H.S., Kogut, M.H., Kim, W.K., Maurer, J.J., Pedroso, A., Lee, M.D., Collett, S.R., Johnson, T.J. and Cox, N.A., 2014. The chicken gastrointestinal microbiome. FEMS microbiology letters, 360(2), pp.100-112.
Osellame, L.D., Blacker, T.S., Duchen, M.R., 2012. Cellular and molecular mechanisms of mitochondrial function. Best Practice and Research, Clinical Endocrinology and Metabolism, 26, 711–723. 10.1016/j.beem.2012.05.003.
Pastorelli, L., De Salvo, C., Mercado, J.R., Vecchi, M., Pizarro, T.T., 2013. Central role of the gut epithelial barrier in the pathogenesis of chronic intestinal inflammation: lessons learned from animal models and human genetics. Frontiers in Immunology, 4, 280. 10.3389/fimmu.2013.00280.
Peralta, M.F., Magnoli, A., Alustiza, F., Nilson, A., Miazzo, R., Vivas, A., 2017. Gut-associated lymphoid tissue: A key tissue inside the mucosal immune system of hens immunized with Escherichia coli F4. Frontiers in Immunology, 8, 568. 10.3389/fimmu.2017.00568.
Petrone-Garcia, V.M., Lopez-Arellano, R., Patiño, G.R., Rodríguez, M.A.C., Hernandez-Patlan, D., Solis-Cruz, B., Hernandez-Velasco, X., et al., 2021. Curcumin reduces enteric isoprostane 8-iso-PGF2α and prostaglandin GF2α in specific pathogen-free Leghorn chickens challenged with Eimeria maxima. Scientific Reports, 11, 1-9. 10.1038/s41598-021-90679-5.
Porter Jr, R.E., 1998. Bacterial enteritides of poultry. Poultry Science, 77, 1159–1165. 10.1093/ps/77.8.1159.
Qureshi, M., 2003. Avian macrophage and immune response: An overview. Poultry Science, 82, 691–698. 10.1093/ps/82.5.691.
Rao, J.N., Wang, J.Y., 2010. Role of GI hormones on gut mucosal growth,. in: Regulation of Gastrointestinal Mucosal Growth. (Morgan & Claypool Life Sciences: San Rafael, CA), 21-33. https://www.ncbi.nlm.nih.gov/books/NBK54093/
Rigottier-Gois, L., 2013. Dysbiosis in inflammatory bowel diseases: the oxygen hypothesis. The ISME Journal, 7, 1256–1261. 10.1038/ismej.2013.80.
Rimet, C.S., Maurer, J.J., Berghaus, R.D., Jordan, B.J., da Silva, L.H.A., Stabler, L.J., et al., 2019. The contribution of Eimeria coinfection and intestinal inflammation to cecal colonization and systemic spread of Salmonella typhimurium deficient in tetrathionate reductase or type III secretion systems Salmonella pathogenicity island 1 or 2. Avian Diseases, 63, 559-567. 10.1637/aviandiseases-D-19-00082.
Rose, M.E., Long, P.L., Bradley, J.W., 1975. Immune responses to infections with coccidia in chickens: gut hypersensitivity. Parasitology, 71, 357–368. 10.1017/s0031182000047132.
Rostagno, M.H., 2020. Effects of heat stress on the gut health of poultry. Journal of Animal Science, 98, p.skaa090. 10.1093/jas/skaa090.
Rubartelli, A., Lotze, M.T., 2007. Inside, outside, upside down: damage-associated molecular-pattern molecules (DAMPs) and redox. Trends In Immunology, 28, 429–436. 10.1016/j.it.2007.08.004.
Ruff, J., Barros, T. L. Tellez Jr, G. Blankenship, J. Lester, H. Graham, B.D., et al., 2020. Research Note: Evaluation of a heat stress model to induce gastrointestinal leakage in broiler chickens. Poultry Science, 99, 1687–1692. 10.1016/j.psj.2019.10.075.
Ruff, J., Tellez, G., Forga, A.J., Señas-Cuesta, R., Vuong, C.N., Greene, E.S., et al., 2021. Evaluation of three formulations of essential oils in broiler chickens under cyclic heat stress. Animals, 11, 1084. 10.3390/ani11041084.
Ruppin, H., Bar-Meir, S., Soergel, K.H., Wood, C.M. and Schmitt Jr, M.G., 1980. Absorption of short-chain fatty acids by the colon. Gastroenterology, 78, 1500-1507. 10.1016/S0016-5085(19)30508-6.
Sagan, L., 1967. On the origin of mitosing cells. Journal of Theoretical Biology, 14, 225–274. 10.1016/0022-5193(67)90079-3.
Sakamoto, K., Hirose, H., Onizuka, A.,Hayashi, M., Futamura, N., Kawamura, Y., Ezaki, T., 2000. Quantitative study of changes in intestinal morphology and mucus gel on total parenteral nutrition in rats. The Journal of Surgical Research, 94, 99–106. 10.1006/jsre.2000.5937.
Salminen, S., Isolauri, E., 2006. Intestinal colonization, microbiota, and probiotics. The Journal of Pediatrics, 149, S115–S120. 10.1016/j.jpeds.2006.06.062.
Saraiva, M., Rodrigues Alves, L.B., Monte, D.F.M., Ferreira, T., Benevides, V.P., Barbosa, F.O., et al., 2021. Deciphering the role of ttrA and pduA genes for Salmonella enterica serovars in a chicken infection model. Avian Pathology, 50 ,257–268. doi: 10.1080/03079457.2021.1909703.
Schneider, S., Wright, C.M., Heuckeroth, R.O., 2019. Unexpected roles for the second brain: enteric nervous system as master regulator of bowel function. Annual Review of Physiology, 81, 235-259. 10.1146/annurev-physiol-021317-121515.
Schulzke, J.D., Ploeger, S., Amasheh, M., Fromm, A., Zeissig, S., Troeger, H., Richter, J., Bojarski, C., Schumann, M., Fromm, M., 2009. Epithelial tight junctions in intestinal inflammation. Annals of the New York Academy of Sciences, 1165, 294–300. 10.1111/j.1749-6632.2009.04062.x.
Sears, C.L., Kaper, J.B., 1996. Enteric bacterial toxins: mechanisms of action and linkage to intestinal secretion. Microbiological Reviews, 60, 167–215. 10.1128/mr.60.1.167-215.199.
Sekirov, I., Russell, S.L., Antunes, L.C.M., Finlay, B.B., 2010. Gut microbiota in health and disease. Physiological Reviews, 90, 859–904. 10.1152/physrev.00045.2009.
Selye, H., 1975. Confusion and controversy in the stress field. Journal of Human Stress, 1, 37-44. 10.1080/0097840X.1975.9940406.
Sender, R., Fuchs, S., Milo, R., 2016. Revised estimates for the number of human and bacteria cells in the body. PLoS Biology, 14, e1002533. 10.1371/journal.pbio.1002533.
Sharma, R., Tepas III, J.J., Hudak, M.L., Mollitt, D.L., Wludyka, P.S., Teng, R.-J., Premachandra, B.R., 2007. Neonatal gut barrier and multiple organ failure: role of endotoxin and proinflammatory cytokines in sepsis and necrotizing enterocolitis. Journal of Pediatric Surgery, 42, 454–461. 10.1016/j.jpedsurg.2006.10.038.
Sharma, R., Young, C., Neu, J., 2010. Molecular modulation of intestinal epithelial barrier: contribution of microbiota. Journal of Biomedicine and Biotechnology, 2010, 305879. 10.1155/2010/305879.
Sherwin, E., Rea, K., Dinan, T.G., Crya, J.F., 2016. A gut (microbiome) feeling about the brain. Current Opinion in Gastroenterology, 32, 96–102. 10.1097/MOG.0000000000000244.
Shehata, A. A., Yalçın, S., Latorre, J. D., Basiouni, S., Attia, Y. A., Abd El-Wahab, et al., 2022a. Probiotics, prebiotics, and phytogenic substances for optimizing gut health in poultry. Microorganisms, 10, 395. 10.3390/microorganisms10020395.
Shehata, A.A., Attia, Y., Khafaga, A.F., Farooq, M.Z., El-Seedi, H.R., Eisenreich, W. and Tellez-Isaias, G., 2022b. Restoring healthy gut microbiome in poultry using alternative feed additives with particular attention to phytogenic substances: Challenges and prospects. Ger. J. Vet. Res, 2, pp.32-42.
Shivaramaiah, C., Barta, J.R., Hernandez-Velasco, X., Téllez, G., Hargis, B.M., 2014. Coccidiosis: recent advancements in the immunobiology of Eimeria species, preventive measures, and the importance of vaccination as a control tool against these Apicomplexan parasites. Veterinary Medicine, 5, 23-34. 10.2147/VMRR.S57839.
Slavich, G.M., Irwin, M.R., 2014. From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychological Bulletin, 140, 774-815. 10.1037/a0035302.
Smatti, M.K., Cyprian, F.S., Nasrallah, G.K., Al Thani, A.A., Almishal, R.O., Yassine, H.M., 2019. Viruses and autoimmunity: a review on the potential interaction and molecular mechanisms. Viruses, 11, 762. 10.3390/v11080762.
Solis-Cruz, B., Hernandez-Patlan, D., Petrone, V.M., Pontin, K.P., Latorre, J.D., Beyssac, E., et al., (2019). Evaluation of a Bacillus-based direct-fed microbial on aflatoxin B1 toxic effects, performance, immunologic status, and serum biochemical parameters in broiler chickens. Avian Diseases, 63, 659–669. 10.1637/aviandiseases-D-19-00100.
Soutter, F., Werling, D., Kim, S., Pastor-Fernández, I., Marugán-Hernández, V., Tomley, F.M., et al., 2021. Impact of Eimeria tenella oocyst dose on parasite replication, lesion score and cytokine transcription in the caeca in three breeds of commercial layer chickens. Frontiers in Veterinary Science, 8, 640041. 10.3389/fvets.2021.640041.
Stecher, B., 2015. The roles of inflammation, nutrient availability and the commensal microbiota in enteric pathogen infection. Microbiology Spectrum, 3, 3.3. 10.1128/microbiolspec.MBP-0008-2014.
Steed, E., Balda, M.S., Matter, K., 2010. Dynamics and functions of tight junctions. Trends in Cell Biology, 20, 142–149. 10.1016/j.tcb.2009.12.002.
Sulejmanovic, T., Liebhart, D., Hess, M., 2013. In vitro attenuated Histomonas meleagridis does not revert to virulence, following serial in vivo passages in turkeys or chickens. Vaccine, 31, 5443–5450. 10.1016/j.vaccine.2013.08.098.
Sulejmanovic, T., Bilic, I., Hess, M., Liebhart, D., 2016. An in vitro attenuated strain of Histomonas meleagridis provides cross-protective immunity in turkeys against heterologous virulent isolates. Avian Pathology, 45, 46-53. 10.1080/03079457.2015.1117057.
Sun, L., Wang, X., Saredy J., Yuan, Z., Yang, X., Wang, H., 2020. Innate-adaptive immunity interplay and redox regulation in immune response. Redox Biology, 37, 101759. 10.1016/j.redox.2020.101759.
Teirlynck, E., Bjerrum, L., Eeckhaut, V., Huygebaert, G., Pasmans, F.. Haesebrouck, F., Dewulf, J., Ducatelle, R., Van Immerseel, F., 2009. The cereal type in feed influences gut wall morphology and intestinal immune cell infiltration in broiler chickens. British Journal of Nutrition, 102, 1453–1461. 10.1017/S0007114509990407.
Teirlynck, E., Gussem, M., Dewulf, J., Haesebrouck, F., Ducatelle, R., Van Immerseel, F., 2011. Morphometric evaluation of “dysbacteriosis” in broilers. Avian Pathology, 40, 139–144. 10.1080/03079457.2010.543414.
Tellez, G., Latorre, J.D., Kuttappan, V.A., Kogut, M.H., Wolfenden, A., Hernandez-Velasco, X., et al., 2014. Utilization of rye as energy source affects bacterial translocation, intestinal viscosity, microbiota composition, and bone mineralization in broiler chickens. Frontiers in Genetics, 5, 339. 10.3389/fgene.2014.00339.
Tellez, G., Latorre, J.D., Kuttappan, V.A., Hargis, N.M., Hernandez-Velasco, X., 2015. Rye affects bacterial translocation, intestinal viscosity, microbiota composition and bone mineralization in turkey poults. PLoS One, 10, e0122390. 10.1371/journal.pone.0122390.
Tellez-Isaias, G., Christine, N., Brittany, D., Callie, M., Lucas, E., Roberto, S, et al., 2021. Developing probiotics, prebiotics, and organic acids to control Salmonella spp. in commercial turkeys at the University of Arkansas USA. German Journal of Veterinary Research, 1, 7–12. 10.51585/gjvr.2021.3.0014.
Tellez Jr, G., Arreguin-Nava, M., Maguey, J., Michel, M., Latorre, J., Merino-Guzman, R., et al., 2020. Effect of Bacillus-direct-fed microbial on leaky gut, serum peptide YY concentration, bone mineralization, and ammonia excretion in neonatal female turkey poults fed with a rye-based diet. Poultry Science, 99, 4514–4520. 10.1016/j.psj.2020.06.018.
Thøfner, I.C.N., Liebhart, D., Hess, M., Schou, T.W., Hess, C., Ivarsen, E., et al., 2012. Antihistomonal effects of artemisinin and Artemisia annua extracts in vitro could not be confirmed by in vivo experiments in turkeys and chickens. Avian Pathology, 41, 487–496. 10.1080/03079457.2012.714459.
Tlaskalová-Hogenová, H., Stvepánková, R., Hudcovic, T., Tuvcková, L., Cukrowska, B., Lodinová-Zádniková, R., et al., 2004. Commensal bacteria (normal microflora), mucosal immunity and chronic inflammatory and autoimmune diseases. Immunology Letters, 93, 97–108. 10.1016/j.imlet.2004.02.005.
Torres-Rodriguez, A., Higgins, S., Vicente, J., Wolfenden, A., Gaona-Ramirez, G., Barton, J., et al., 2007. Effect of lactose as a prebiotic on turkey body weight under commercial conditions. J. Appl. Poult. Res. 16:635–641. doi: 10.3382/japr.2006-00127
Ulluwishewa, D., Anderson, R.C., McNabb, W.C., Moughan, P.J., Wells, J.M., Roy, N.C., 2011. Regulation of tight junction permeability by intestinal bacteria and dietary components. The Journal of Nutrition, 141, 769–776. 10.3945/jn.110.135657.
Van der Hoeven-Hangoor, E., Rademaker, C., Paton, N., Verstegen, M., Hendriks, W., 2014. Evaluation of free water and water activity measurements as functional alternatives to total moisture content in broiler excreta and litter samples. Poultry Science, 93, 1782–1792. 10.3382/ps.2013-03776
Vicuña, E., Kuttappan, V., Galarza-Seeber, R., Latorre, J., Faulkner, O., Hargis, B., et al., 2015. Effect of dexamethasone in feed on intestinal permeability, differential white blood cell counts, and immune organs in broiler chicks. Poultry Science, 94, 2075-2080. 10.3382/ps/pev211.
Vighi, G., Marcucci, F., Sensi, L., Di Cara, G., Frati, F., 2008. Allergy and the gastrointestinal system. Clinical and Experimental Immunology, 153, 3–6. 10.1111/j.1365-2249.2008.03713.x.
Vuong, C.N., Mullenix, G.J., Kidd, M.T., Bottje, W.G., Hargis, B.M., Tellez-Isaias, G., 2021. Research Note: Modified serum fluorescein isothiocyanate dextran (FITC-d) assay procedure to determine intestinal permeability in poultry fed diets high in natural or synthetic pigments. Poultry Science, 100, 101138. 10.1016/j.psj.2021.101138.
Wallis, J.W., Aerts, J., Groenen, M.A., Crooijmans, R.P., Layman, D., Graves, T.A., et al., 2004. A physical map of the chicken genome. Nature, 432, 761–764. 10.1038/nature03030.
Weiss, G.A., Hennet, T., 2017. Mechanisms and consequences of intestinal dysbiosis. Cell and Molecular Life Science, 74, 2959–2977. 10.1007/s00018-017-2509-x.
Wickramasuriya, S.S., Park, I., Lee, K., Lee, Y., Kim, W.H., Nam, H., Lillehoj, H.S., 2022. Role of physiology, immunity, microbiota, and infectious diseases in the gut health of poultry. Vaccines, 10, 172. 10.3390/vaccines10020172.
Wideman, R.F., Prisby, R.D., 2011. Bone circulatory disturbances in the development of spontaneous bacterial chondronecrosis with osteomyelitis: a translational model for the pathogenesis of femoral head necrosis. Frontiers in Endocrinology, 3, 183–183. doi: 10.3389/fendo.2012.00183.
Wideman, R., Al-Rubaye, A., Kwon, Y., Blankenship, J., Lester, H., Mitchell, H., Pevzner, I., Lohrmann, T., Schleifer, J., 2015. Prophylactic administration of a combined prebiotic and probiotic, or therapeutic administration of enrofloxacin, to reduce the incidence of bacterial chondronecrosis with osteomyelitis in broilers. Poultry Science, 94, 25–36. 10.3382/ps/peu025.
Williams, R., 2005. Intercurrent coccidiosis and necrotic enteritis of chickens: rational, integrated disease management by maintenance of gut integrity. Avian Pathology, 34, 159–180. 10.1080/03079450500112195.
Wink, D.A., Hines, H.B., Cheng, R.Y., Switzer, C.H., Flores-Santana, W., Vitek, M.P., et al., 2011. Nitric oxide and redox mechanisms in the immune response. Journal of Leukocite Biology, 89, 873–891. 10.1189/jlb.1010550.
Winter, S.E., Keestra, A.M., Tsolis, R.M., Bäumler, A.J., 2010a. The blessings and curses of intestinal inflammation. Cell Host and Microbe, 8, 36–43. 10.1016/j.chom.2010.06.003
Winter, S.E., Thiennimitr, P, Winter, M.G., Butler, B.P., Huseby, D.L., Crawford, R.W., et al., 2010b. Gut inflammation provides a respiratory electron acceptor for Salmonella. Nature, 467, 426–429. 10.1038/nature09415.
Wu, R.Y., Määttänen, P., Napper, S., Scruten, E., Li, B., Koike, Y., et al., 2017. Non-digestible oligosaccharides directly regulate host kinome to modulate host inflammatory responses without alterations in the gut microbiota. Microbiome, 5, 135. 10.1186/s40168-017-0357-4.
Yamamoto, M.L., Maier, I., Dang, A.T., Berry, D., Liu, J., Ruegger, P.M., Yang, J.I., Soto, P.A., Presley, L.L., Reliene, R., Westbrook, A.M., 2013. Intestinal bacteria modify lymphoma incidence and latency by affecting systemic inflammatory state, oxidative stress, and leukocyte genotoxicity. Cancer Research, 73, 4222-4232. 10.1158/0008-5472.CAN-13-0022.
Yegani, M., Korver, D., 2008. Factors affecting intestinal health in poultry. Poultry Science, 87, 2052–2063. 10.3382/ps.2008-00091.
Yerpes, M., P. Llonch, and X. Manteca. 2020. Factors associated with cumulative first-week mortality in broiler chicks. Animals, 10, 310.
Yerpes, M., Llonch, P., Manteca, X. 2021. Effect of environmental conditions during transport on chick weight loss and mortality. Poultry Science, 100, 129–137. 10.1016/j.psj.2020.10.003. Epub 2020 Oct 10.
Yeoman, C.J., Chia, N., Jeraldo, P., Sipos, M., Goldenfeld, N.D. and White, B.A., 2012. The microbiome of the chicken gastrointestinal tract. Animal health research reviews, 13(1), pp.89-99.
Zhu, B., Wang, X., Li, L., 2010. Human gut microbiome: the second genome of human body. Protein and Cell 1, 718-725. 10.1007/s13238-010-0093-z
Zou, Y., Xiang, Q., Wang, J., Peng, J., Wei, H., 2016. Oregano essential oil improves intestinal morphology and expression of tight junction proteins associated with modulation of selected intestinal bacteria and immune status in a pig model. BioMed Research International, 2016, 5436738. 10.1155/2016/5436738.
Zou, X., Ji, J., Wang, J., Qu, H., Shu, D.M., Guo, F.Y., et al., 2018. Dextran sulphate sodium (DSS) causes intestinal histopathology and inflammatory changes consistent with increased gut leakiness in chickens. British Poultry Science, 59, 166-172. 10.1080/00071668.2017.1418498.



















.jpg&w=3840&q=75)