The value of understanding total productivity
The principal objective of any enterprise is to optimize profitability (return on investment) over the long term. Poultry production is highly competitive, dictating high efficiency of conversion of resources into salable products that are acceptable to consumers. The production of poultry meat and eggs is achieved in the context of either vertical or horizontal integration with the chain extending from primary breeding to point-of-sale (Laughlin, 2001).
Efficiency can be measured at each of the component steps in the process, and intermediate and transfer prices can be derived for comparison with budgetary values and standards. Similarly, quality can be quantified and food safety, an emerging criterion, can be monitored by application of HACCP systems. Each component of the production process can be evaluated by cost and contribution. In a market-driven enterprise value is regarded as a function of quality, yield and cost of production with an emphasis shifting from performance to profit through the chain of realization (Figure 1).
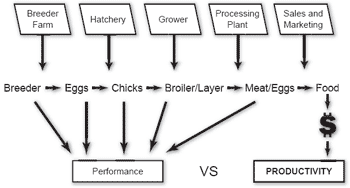
Figure 1. Performance versus productivity.
It is emphasized that evaluation of individual components in isolation can be deleterious to the enterprise. Sub-optimization often introduces distortions in allocation of resources. Achieving economies in one segment may be deleterious to production efficiency, cost or quality in subsequent components. Examples include relaxing the criteria for chick viability during culling to boost either hatchability or the number of chicks produced per unit of labor cost. This approach, although seemingly beneficial to the hatchery, will result in an elevation in first-week mortality, reflecting adversely on measures of efficiency in broiler production.
Injudicious administration of antibiotics to suppress mortality in broilers may result in drug residues and will contribute to the emergence of drug-resistant organisms with implications for both quality and food safety. Relaxing standards for recruitment and reducing training or exceeding design limitations on equipment, will lead to industrial accidents, breakdowns and deterioration in quality. Constant pursuit of short-term savings may be reflected in both intermediate and long-term erosion of profitability.
Improving profitability
Since feed represents the largest single cost of production, conversion of feed ingredients into salable eggs and poultry meat is pivotal to optimal profitability. During the 1970s and 1980s, least-cost formulation of feed was facilitated by the availability of inexpensive computer software and user-friendly analytical equipment. More recently the emphasis in nutrition has shifted to least-cost production of salable product. Incorporation of an enzyme supplement into diets marginally increases the cost per ton of feed but generates benefits from enhanced digestibility, improved growth or egg production.
Controlled experiments and structured trials on enzymes confirm an incremental return over the cost of these additives. Expenditures on biosecurity and appropriate vaccines generate high benefit-tocost ratios, justifying expenditure on measures to diagnose and prevent infection.
IMPROVING PRODUCTIVITY
It is axiomatic that the genetic potential of broiler, turkey and egg production breeds (genotype) exceeds the production parameters obtained under commercial conditions. Restraints to production efficiency include variability in the nutritional quality of ingredients, exposure to disease, climatic extremes inducing physiological stress and inappropriate management of feeding, drinking and ventilation systems. Disharmony between the flock and the environment will result in diversion of protein and energy into non-productive metabolic activities that detract from potential production standards and frequently add to the cost of salable product (Humphrey et al., 2002; Beck, 1996).
Flock uniformity and rapid growth rate in broilers and turkeys or egg production in breeders and commercial hens are regarded as measures of production efficiency.
Flock health remains the most important contributor to optimal performance. Diseases that serve as rate-limiting factors to efficiency range in their impact from catastrophic infections such as velogenic Newcastle disease and highly pathogenic avian influenza, through to erosive conditions including colibacillosis and mycoplasmosis. The clinical effects and subsequent financial impact of disease can be influenced by concurrent environmental stress, nutritional adequacy and husbandry.
Nutritionists, veterinarians and production management should function as a team to prevent disease, provide adequate diets and an environment that narrows the gap between genotype and phenotype. Innovations in biotechnology including synthesis of feed additive enzymes, bioplexed minerals, genetically engineered vaccines and fermentation products that enhance performance all contribute to productivity.
Challenges facing the poultry industry
The poultry industries of the significant poultry meat and egg-producing nations have achieved high levels of efficiency through integration. Concentration of flocks in limited geographic areas leads to higher risks from disease challenge and disposal of waste.
In addition, large integrations compete for available food ingredients, labor and water and place pressure on the existing transport and communications infrastructure.
Current issues which impact both productivity and profitability include cost efficiency, product quality and food safety.
COST EFFICIENCY
The availability of enzymes derived from microbial and fungal organisms offers the potential to enhance the digestibility of both standard and unconventional ingredients, which has important consequences for health and productivity. Initially use of exogenous enzymes focused on digestive problems such as increased viscosity of digesta due to presence of β-glucans in barley or pentosans in wheat. Such applications for enzymes allow higher rates of inclusion of economical ingredients and solve problems inherent in using new-crop cereals.
More recently, application of enzymes has been expanded to address standard vegetable protein sources, especially soybean meal and full-fat soy.
The need to find ways to reduce the impact of antinutritive factors such as oligosaccharides in soy is receiving increased emphasis because of the declining availability and increasing cost of animal protein ingredients, including fish meal.
AllzymeTM Vegpro, an enzyme targeted to the complex protein-oligosaccharide matrix of oil meals, has been shown to improve digestion and utilization of vegetable protein ingredients by 3-7% (Rostagno, 2000; see Schange et al., 2003 in this volume). This is further improved in animals with restricted digestive capabilities, be they young animals with immature digestive systems, or older animals that have been compromised by disease. Such a strategy for enhancing nutrient utilization fits well with related goals for reducing prevalence and consequence of both subclinical and clinical enteritis (reduced protein through-flow).
Alltech’s AllzymeTM Phytase, produced by solid state fermentation, enhances utilization of phosphorus bound to phytate in wheat-soy and cornsoy broiler diets (Ravindran et al., 2001; Ravindran et al., 1999; Harter-Dennis et al., 2001; Roland et al., 2000). During the period 1 through 21 days of age, supplemental phytase is equivalent in nutrient content to 0.25% dietary phosphorus (Harter- Dennis et al., 2001; Table 1). Under practical conditions increased weight gain is associated with higher feed intake and more favorable feed conversion. Improved performance may also be attributed to the range of enzyme activities inherent to enzyme products produced via solid state fermentation (Harter-Dennis et al., 2001; Ravindran et al., 2001).
Table 1. Beneficial effect of AllzymeTM Phytase on broiler performance.
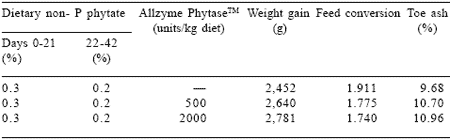
Harter-Dennis et al., 2001
Health can be enhanced by inclusion of compounds in the diet that enhance the function of the immune system and modify intestinal flora (Finucane et al., 1999; Cotter et al., 2002; Sisak, 1995). Cell wall extracts of Saccharomyces cerevisiae are available commercially as Bio-Mos® to improve feed conversion efficiency in chicks and poults. Bio-Mos® alters the composition of intestinal flora, resulting in increased numbers of goblet cells in the mucosa and an increase in the depth of the intestinal crypts (Ferket et al., 2002; Savage et al., 1997). Decreased rate of turnover of enterocytes is reflected in improved growth rate and feed conversion efficiency in both broilers and turkeys (Hooge, 2003; Sefton and Collett, 2002; Table 2).
Inclusion of Bio-Mos® in diets at 1 g/kg elevates both IgG and IgA in the serum of 7-week old turkey poults (Savage et al., 1996). Electrophoresis demonstrates significantly increased levels of the two immunoglobulins, denoting stimulation of humoral immunity. This systemic response can be correlated with increased growth rate and financial contribution.
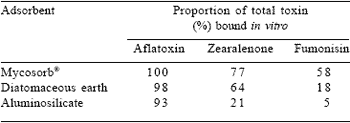
Mycotoxins detract significantly from optimal performance. Immuno-based analytical systems are extremely sensitive and proportionately specific for aflatoxins and fusariotoxins. Variability in the distribution of toxins in large consignments of ingredients results in significant sampling errors, which can obscure low-grade and subclinical effects. Retardation in growth and immunosuppression resulting in elevated mortality and downgrading, may be attributed to combinations of mycotoxins at low dietary inclusion levels. Efficient adsorbents of mycotoxins can restore productivity to accepted levels at minimal cost. Mycosorb®, derived from an extract of the glucan-rich inner cell wall of Saccharomyces cerevisiae, has proven effective under both experimental and commercial conditions against a wide range of toxins, exceeding the relatively limited binding capacity of bentonite clays and both synthetic and natural zeolites (Kubena et al., 1993; Philips et al., 1988; Smith, 2001; Tables 3 and 4).
Table 2. Effect of Bio-Mos® on broiler performance.
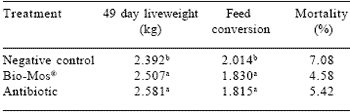
abMeans in columns without common superscripts differ (P<0.05)
Table 3. Amelioration of mycotoxicosis effects by Mycosorb® addition to diets fed turkey poults.
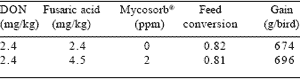
Table 4. Comparative adsorbent ability of three commercially available adsorbents.
ENHANCED BREEDER PERFORMANCE
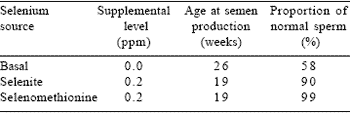
Substitution of natural selenium compounds for selenite in micronutrient supplements for broiler parents results in enhanced egg production in hens and increased fertility associated with improved semen quality in cockerels (Edens, 2002) (Table 5). The importance of selenium in a wide range of antioxidant functions underscores the necessity of supplying selenium in a form that is metabolically useful for optimum growth and efficiency (Naylor et al., 2000) (Table 6).
Table 5. Effect of substituting sodium selenite with Sel-PlexTM organic selenium on male reproductive development.
E dens, 2002
Table 6. Comparative effects of inorganic selenium and Sel- PlexTM on broiler performance and mortality.

Naylor et al., 2000
PRODUCT QUALITY
Beneficial conversion of resources including feed ingredients to salable product of acceptable quality is exemplified by substitution of selenoamino acidcontaining Sel-PlexTM for inorganic selenium in poultry diets. Drip loss, an important determinant of quality, which can be evaluated by the consumer, can be reduced by inclusion of a source of organic selenium in diets (Edens, 1996; Naylor et al., 2000; Downs et al., 2000).
Supplementing diets for laying hens with Sel- PlexTM will result in significantly higher levels of available selenium in eggs (Paton, 2002; Surai, 2002) (Table 7). ‘Designer eggs’ currently represent the fastest-growing segment of the commercial egg market (Shane, 2001). Enrichment of hen diets with folic acid, organic selenium and ω-3 fatty acids generate high returns over the incremental cost of nutritional supplements, enhancing the return on investment compared to production and marketing of generic eggs.
Table 7. Comparative effects of inorganic selenium and Sel- Plex™ on selenium content of eggs.

FOOD SAFETY
Recent outbreaks of foodborne infection in the US and other industrialized countries have stimulated considerable consumer and regulatory pressure on producers to reduce levels of E. coli, Salmonella, Campylobacter and Listeria. Emerging drug resistance attributed to feeding growth-stimulating levels of tetracyclines, streptogramins and macrolides has resulted in increasingly stringent control. In the case of the EU, an outright ban has been imposed on four widely used feed additives (Ratcliff, 2001). Withdrawal of antibiotic growth stimulants, which suppress Gram-positive components of the intestinal flora, may result in outbreaks of clostridial enteritis, gangrenous dermatitis and botulism (Finucane et al., 1999; Hofacre, 2001; Spring, et al., 2000; Newman et al., 1995). Every effort to support integrity of intestinal tissues and the animal’s disease resistance mechanisms must be made.
ENVIRONMENTAL ISSUES
Release of phosphorus into litter with subsequent eutrophication of waterways has resulted in regional and state control regulations. Approximately twothirds of plant phosphorus, which is bound to phytic acid, is unavailable to monogastric animals, requiring supplementation of diets with inorganic phosphorus.
Natural microbial phosphatase subsequently releases phosphorus from phytic acid in litter. This results in high levels of application of the element to agricultural land, with ultimate transfer to waterways. Regulatory authorities in regions with intensive poultry production have mandated addition of phytase to poultry diets to reduce intake and hence excretion of phosphorus. Studies conducted in the US and New Zealand have demonstrated benefits of phytase supplementation including improved weight gain, feed conversion efficiency and skeletal integrity while reducing added dietary phosphorus (Ravindran et al., 1999).
Acknowledgments
The author gratefully acknowledges the help of Dr. Simon Shane in preparation of this manuscript.
References
Author: STEPHEN R. COLLETTBeck, M.A. 1996. The role of nutrition in viral disease. Nutri. Biochem. 7:683-690.
Cotter, P.F., A.E. Sefton and M.S. Lilburn. 2002. Manipulating the immune system of layers and breeders: novel applications of mannan oligosaccharides. In: Nutritional Biotechnology in the Feed and Food Industry. (T.P. Lyons and K.A. Jacques, eds.). Nottingham University Press, Nottingham, UK, pp. 21-28.
Downs, K.M., J.B. Hess and S. Bilgili. 2000. Selenium source. Effect on broiler carcass characteristics, meat quality and drip loss. J. Appl. Anim. Res. 18:61-72.
Edens, F.W. 1996. Organic Selenium: from feathers to muscles integreity to drip loss five years onward: no more sodium selenite! In: Biotechnology in the Feed Industry, Proceedings of the 12th Annual Symposium, (T.P. Lyons and K.A. Jacques, eds.), Nottingham University Press, Nottingham, UK.
Edens, F.W. 2002 Practical applications for selenomethionine: broiler breeder reproduction. In: Nutritional Biotechnology in the Feed and Food Industry. (T.P. Lyons and K.A. Jacques, eds.). Nottingham University Press, Nottingham, UK, pp. 29-42.
Ferket, P.R., C.W. Parks and J.L. Grimes 2002. Mannan oligosaccharides versus antibiotics for turkeys. In: Nutritional Biotechnology in the Feed and Food Industry. (T.P. Lyons and K.A. Jacques, eds.). Nottingham University Press, Nottingham, UK, pp. 43-64.
Finucane, M., K.A. Dawson, P. Spring and K.E. Newman. 1999. Effects of Mannanoligosaccharide and BMD on Gut Microflora of Turkey Poults. Poult. Sci. 78 (Suppl. 1):77.
Finucane, M., P. Spring and K.E. Newman. 1999b. Incidence of mannose-sensitive adhesions in enteric bacteria. Poult. Sci. 78(Suppl. 1):139.
Harter-Dennis, J.M. 2000. Phytase application variations in broiler diets and legislative update. In: Biotechnology in the Feed Industry. (T.P. Lyons and K.A. Jacques, eds.). Nottingham University Press, Nottingham, UK, pp. 163-174.
Harter-Dennis, J.M, J. Timmons and J. Driver. 2001. Effect of application variation and side activities on the efficacy of phytase in broiler diets. In: Biotechnology in the Feed Industry. (T.P. Lyons and K.A. Jacques, eds.). Nottingham University Press, Nottingham, UK, pp. 241-253.
Hofacre, C.L. 2001. Necrotic enteritis, currently a billion dollar disease: is there anything new on the horizon? In: Biotechnology in the Feed Industry. (T.P. Lyons and K.A. Jacques, eds.). Nottingham University Press, Nottingham, UK, pp. 79-86.
Hooge D.M. 2003. Nutrition and Health: Poultry – Broiler chicken performance may improve with MOS. Feedstuffs January 6. pp. 11-13.
Humphrey, B.D., E.A. Koutsos and K.C. Klassing 2002. Requirements and priorities of the immune system for nutrients. In: Nutritional Biotechnology in the Feed and Food Industry. (T.P. Lyons and K.A. Jacques, eds.). Nottingham University Press, Nottingham, UK, pp. 69-77.
Kubena, L.F., R.B. Harvey, W.E. Huff, M.E. Elissalde, A.G. Yersin, T.D. Phillips and G.E. Rottinghaus. 1993. Efficacy of hydrated sodium calcium aluminosilicate to reduce the toxicity of aflatoxin and diacetoxyscripenol. Poult. Sci. 72:51- 59.
Laughlin, K.F. 2001. Achieving sustained rapid growth in the modern broiler chicken – a breeding company perspective. Poultry Beyond 2005. 2nd International Poultry Broiler Nutritionists’ conference. Rotorua, New Zealand. 107-112.
Naylor, A.J., M. Choct and K.A. Jacques. 2000. Effects of selenium source and level on performance and meat quality in male broilers. Poult. Sci. 79(Suppl. 1):117.
Newman, K.E., 2000. The biochemistry behind esterified glucomannans: titrating mycotoxins out of the diet. In: Biotechnology in the Feed Industry. (T.P. Lyons and K.A. Jacques, eds.). Nottingham University Press, Nottingham, UK, pp. 369-381.
Newman, K.E., P. Spring and L.S. Snitzer. 1995. Effect of thermal treatment on the ability of mannan oligosacchride to adsorb enteric bacteria. J. Anim. Sci. 73(Suppl. 1):310.
Norheim, G. and K. Moksnes. 1985. Distribution and elimination of selenium and glutathione peroxidase (GSH-Px) in chickens after supplementation with sodium selenite or selenomethionine. In: Proceedings: Fifth International Symposium on Trace Elements in Man and Animals. (C.F. Mills, I. Bremmer and J.K. Chesters, eds.). CAB, Farnum Royal, Slough, UK, pp. 493-495.
Paton, N.D., A.H. Cantor, A.J. Pescatore, M.J. Ford and C.A. Smith. 2002. Absorption of developing chick embryos during incubation. In: Nutritional Biotechnology in the Feed and Food Industry. (T.P. Lyons and K.A. Jacques, eds.). Nottingham University Press, Nottingham, UK, pp. 107-122.
Philips, T.D., L.F. Kubena, R.B. Harvey, D.R. Taylor and N.D. Heidelbaugh. 1988. Hydrated sodium calcium aluminosilicate: a high affinity sorbent for aflatoxin. Poult. Sci. 67:243-247.
Ratcliff J. 2001. Current issues and likely challenges beyond 2005. Poultry Beyond 2005. 2nd International Poultry Broiler Nutritionists’ conference. Rotorua, New Zealand. 7-19
Ravindran, V., P.H. Selle and W.L. Bryden. 1999. Effects of phytase supplementation, individually and in combination, with glycanase, on the nutritive value of wheat and barley. Poult. Sci. 78:1588- 1595.
Ravindran, V., Y.B. Wu, D.V. Thomas, B.J. Camden, P.C.H. Morel and W.H. Hendriks. 2001. Improving posphorus availability in broiler diets based on wheat-soybean meal using microbial phytase produced in solid state fermentation. In: Biotechnology in the Feed Industry. (T.P. Lyons and K.A. Jacques, eds.). Nottingham University Press, Nottingham, UK, pp. 255-266.
Roland, D.A., M. Bryant and A. Bateman. 2000. Do non-GMO enzymes work as well as GMO sources? A comparison of phytase sources in low phosphorus diets fed to layers. In: Biotechnology in the Feed Industry. (T.P. Lyons and K.A. Jacques, eds.). Nottingham University Press, Nottingham, UK, pp. 153-161.
Rostagno, H.S., Tejedor, A.A., Albino, L.F.T. and Silva, J.H.V. 2000. Enzyme supplementation of corn/soybean diets improves ileal digestibility of nutrients in broiler chicks. In: Biotechnology in the Feed Industry. (T.P. Lyons and K.A. Jacques, eds.). Nottingham University Press, Nottingham, UK, pp. 175-182.
Savage, T.F., P.F. Cotter and E.I. Zakrzewska. 1996. The effect of feeding a mannanoligosaccharide on immunoglobulin plasma IgA and bile IgA of Wrolstad MW male turkeys. Poult. Sci. 75(Suppl. 1):143.
Savage, T.F., E.I. Zakrzewska and J.R. Andreasen, Jr. 1997. The Effects of Feeding Mannan Oligosaccharide Supplemented Diets to Poults on Performance and the Morphology of the Small Intestine. Poult. Sci. 76 (Suppl. 1):139.
Sefton A.E. and S.R. Collett. 2002. Comparison of survey data on antibiotic growth promotants and response to alternatives. Poster Presentation at Western Poultry Diseases Conference. Porta Vallarta. Mexico
Shane S. M. 2001. Mannan oligosaccharides in poultry nutrition: mechanisms and benefits. In: Biotechnology in the Feed Industry. (T.P. Lyons and K.A. Jacques, eds.). Nottingham University Press, Nottingham, UK, pp. 65-78.
Sisak, F. 1995. Bio-Mos-mediated stimulation of phagocytosis as assessed by luminol-enhanced chemiluminescence. Czech Research Institute. Poster presented at the 11th Annual Symposium on Biotechnology in the Feed Industry. Lexington, KY, April.
Smith, T.K., E.J. MacDonald and S. Haladi. 2001. Current concepts in feed-bourne mycotoxins and the potential for prevention of mycotoxicoses. In: Biotechnology in the Feed Industry. (T.P. Lyons and K.A. Jacques, eds.). Nottingham University Press, Nottingham, UK, pp. 183-190.
Spring, P., C. Wenk, K.A. Dawson and K.E. Newman. 2000. The effects of dietary mannanoligosaccharides on cecal parameters and the concentrations of enteric bacteria in the ceca of Salmonella-challenged broiler chicks. Poult. Sci. 79:205-211.
Surai, P.F. 2002. Selenium in poultry nutrition: a new look at an old element. 1. Antioxidant properties, deficiency and toxicity. World’s Poultry Sci. J. 58:333-347.
Alltech Inc., Nicholasville, Kentucky, USA









.jpg&w=3840&q=75)