Response of Broiler to Supplementation of Human Oral Rehydration Salt during Pre-Slaughter Fasting
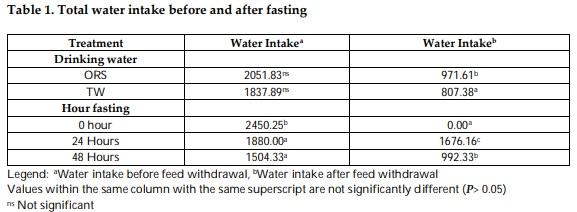
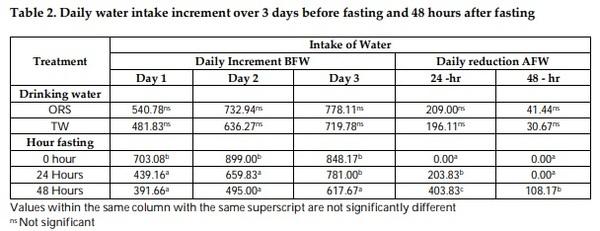
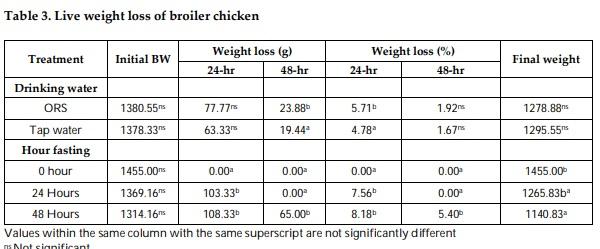
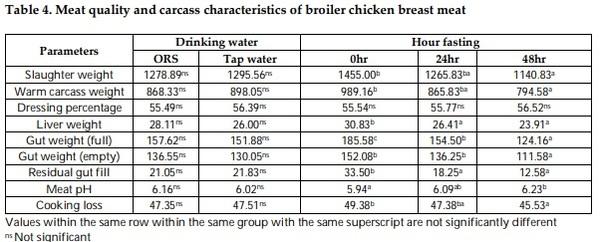
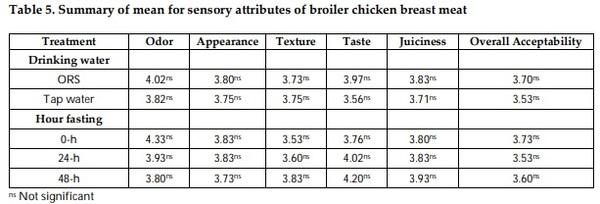
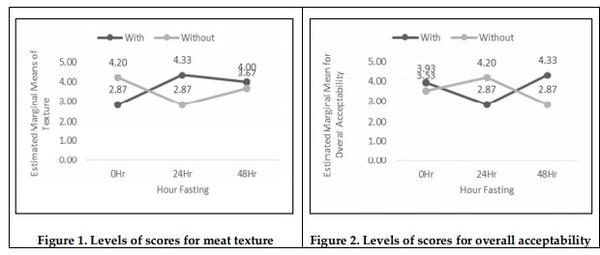
Mitigation measures are means to remunerate the losses by pre-slaughter feed withdrawal practices of broiler chicken. The therapeutic effect of drinking with oral rehydration salt solution (ORSS) versus tap water (TW) during fasting (0-h versus 24-h versus 48-h) period was investigated in this trial. Data on water intake, meat quality, carcass traits and sensory of broiler chicken were subjected to a two-way ANOVA in a complete randomized design. The water intake of broiler was significantly higher before fasting while highly significantly lower after feed removal. Water with ORSS was consumed by broilers significantly higher over TW after feed withdrawal. Live weight loss was significantly higher in ORSS group while consistent significant higher live weight loss in 48-hr fasted chicken in all tested periods of fasting P< 0.05. Slaughter weight, hot carcass, liver weight, and gut (full, empty, and residual) were significantly affected by the fasting period while similar average values in dressing percentage. Mean values of meat pH and cooking loss were statistically significant by fasting while comparable in effect of water. Both drinking water and the fasting period did not alter the meat sensory profile. All parameters tested, drinking water did not interact (P> 0.05) with fasting periods except on texture and overall acceptability of meat sensory. Broilers fasted within 48 hours significantly lost live weight while pH and cooking loss responded positively and moderate acceptability on sensory of meat. ORS therapy significantly induced water intake but negatively affect live weights.
Keywords: Feed Withdrawal, Oral rehydration, Water Intake, Carcass Characteristics, and Meat Quality.
Water intake = Water offered – Water remained
Live weight loss = Pre-fasting weight – Post-fasting weight
% live weight loss = Pre-fasting weight/Post-Fasting weight x 100
Dressing percentage = hot carcass weight/Post-withdrawal weight x 100
Residual gut fill = Digestive tract full – Digestive tract full empty
1. Delezie, E., Swennen, Q., Buyse, J., & Decuypere, E. (2007). The effect of feed withdrawal and crating density in transit on metabolism and meat quality of broilers at slaughter weight. Poultry Science, 86(7), 1414-1423.
2. Smidt, M. J., Formica, S. D., & Fritz, J. C. (1964). Effect of fasting prior to slaughter on yield of broilers. Poultry Science, 43(4), 931-934.
3. FSIS, U. (1996). Pathogen reduction: hazard analysis and critical control point (HACCP) systems; final rule. Federal Register, 61, 38806-38989.
4. Contreras-Castillo, C., Pinto, A. A., Souza, G. L., Beraquet, N. J., Aguiar, A. P., Cipolli, K. M. V. A. B., & Ortega, E. M. (2007). Effects of feed withdrawal periods on carcass yield and breast meat quality of chickens reared using an alternative system. Journal of Applied Poultry Research, 16(4), 613-622.
5. May, K. N., & Brunson, C. C. (1955, January). Effect of length of starvation period on eviscerated yield of broilers. In Poultry Science (Vol. 34, No. 5, pp. 1210-1210). 1111 NORTH DUNLAP AVE, SAVOY, IL 61874: POULTRY SCIENCE ASSOC INC.
6. Veerkamp, C. H. (1978). The influence of fasting and transport on yields of broilers. Poult. Sci, 57(3), 634-638.
7. Lyon, C. E., Papa, C. M., & Wilson Jr, R. L. (1991). Effect of feed withdrawal on yields, muscle pH, and texture of broiler breast meat. Poultry Science, 70(4), 1020-1025.
8. Papa, C.M., 1991. Lower gut contents of broiler chickens withdrawn from feed and held in cages. Poultry Sci. 70:375-380.
9. Moran Jr, E. T., & Bilgili, S. F. (1995). Influence of broiler livehaul on carcass quality and further-processing yields. Journal of Applied Poultry Research, 4(1), 13-22.
10. Rao, M. C. (2004). Oral rehydration therapy: new explanations for an old remedy. Annu. Rev. Physiol., 66, 385-417.
11. Sayed, M. A. M., & Downing, J. (2011). The effects of water replacement by oral rehydration fluids with or without betaine supplementation on performance, acid-base balance, and water retention of heat-stressed broiler chickens. Poultry science, 90(1), 157-167.
12. Siekmann, L. (1985). Determination of creatinine in human serum by isotope dilution-mass spectrometry. Definitive methods in clinical chemistry, IV. Clinical Chemistry and Laboratory Medicine, 23(3), 137-144.
13. Kwanchai, A. G., & Arturo, A. G. (1984). Statistical procedures for agricultural research. John Wailey et Sons: New York.
14. Degen, A. A., Kam, M., Rosenstrauch, A., & Plavnik, I. (1991). Growth rate, total body water volume, dry-matter intake and water consumption of domesticated ostriches (Struthio camelus). Animal Science, 52(1), 225-232.
15. Fairchild, B. D., & Ritz, C. W. (2009). Poultry drinking water primer.
16. Borges, S. A., Ariki, J., de Moraes, V. M. B., Pedroso, A. A., Salvador, D., & Martins, C. L. (2000). Potassium chloride supplementation in broilers diets during summer. Ars Veterinaria, 16(1), 64-70.
17. Ahmad, T., Khalid, T., Mushtaq, T., Mirza, M. A., Nadeem, A., Babar, M. E., & Ahmad, G. (2008). Effect of potassium chloride supplementation in drinking water on broiler performance under heat stress conditions. Poultry science, 87(7), 1276-1280.
18. Wabeck, C. J. (1972). Feed and water withdrawal time relationship to processing yield and potential fecal contamination of broilers. Poultry Science, 51(4), 1119-1121
19. Salmon, R. E. (1979). Effect of food and water deprivation on live-weight shrinkage, eviscerated carcass yield and water absorption during chilling of Turkey carcasses. British Poultry Science, 20(3), 303-306.
20. Greaser, M. L. (1986). Conversion of muscle to meat. Muscle as food, 37-102.
21. Rahi, R. A., Afrin, S., Howlider, M. A. R., & Ali, M. S. (2015). Response of broiler to supplementation of potassium chloride during summer. Asian Journal of Medical and Biological Research, 1(1), 103-108.
22. Weinsier, R. L. (1971). Fasting—a review with emphasis on the electrolytes. The American journal of medicine, 50(2), 233-240.
23. Saffle, R. L., & Cole, J. W. (1960). Fasting effects on dressed yields, shrinkage, and pH of contractile tissue in swine. Journal of Animal Science, 19(1), 242-248.
24. Faucitano, L., Chevillon, P., & Ellis, M. (2010). Effects of feed withdrawal prior to slaughter and nutrition on stomach weight, and carcass and meat quality in pigs. Livestock Science, 127(2-3), 110-114.
25. Beattie, V. E., Burrows, M. S., Moss, B. W., & Weatherup, R. N. (2002). The effect of food deprivation prior to slaughter on performance, behaviour and meat quality. Meat science, 62(4), 413-418.
26. Souza, B. B., Bertechini, A. G., Teixeira, A. S., Lima, J. A. F., Pereira, S. L., & Fassani, E. J. (2002). The effects of potassium and ammonium chlorides on the performance and deposition of abdominal fat in carcass of broilers diets raised in summer. Brazilian Journal of Poultry Science, 4(3), 209-218
27. Jones, S. D. M., Rompala, R. E., & Haworth, C. R. (1985). Effects of fasting and water restriction on carcass shrink and pork quality. Canadian Journal of Animal Science, 65(3), 613-618
28. Warriss, P. D., Kestin, S. C., Brown, S. N., Knowles, T. G., Wilkins, L. J., Edwards, J. E., ... & Nicol, C. J. (1993). The depletion of glycogen stores and indices of dehydration in transported broilers. British Veterinary Journal, 149(4), 391-398.
29. Koreleski, J., Swiatkiewicz, S., & Arczewska, A. (2010). The effect of dietary potassium and sodium on performance, carcass traits, and nitrogen balance and excreta moisture in broiler chicken. Journal of Animal and Feed Sciences, 19(2), 244-256.
30. Castroverde, K. B. V., Olarve, J. P., & Cruzana, B. C. (2010). Effects of feed withdrawal prior to slaughter on broiler's carcass characteristics and meat quality. Philippine Journal of Veterinary Medicine, 47(2), 98-102.
31. Gagnon, M. P., Bissonnette, P., Deslandes, L. M., Wallendorff, B., & Lapointe, J. Y. (2004). Glucose accumulation can account for the initial water flux triggered by Na+/glucose cotransport. Biophysical journal, 86(1), 125-133.
32. Van Laack, R. L. J. M. (2000). Determinants of ultimate pH of meat and poultry. In 53 rd Annual Reciprocal Meat Conference (pp. 74-75).
33. Ngoka, D. A., & Froning, G. W. (1982). Effect of free struggle and pre-slaughter excitement on color of turkey breast muscles. Poultry Science, 61(11), 2291-2293.
34. Price, J. (1971). The science of meat and meat products. American Meat Institute Foundation.
35. Monin, G. (1988). Post-mortem evolution of muscle tissue and consequences on the quality of pork.
36. Warner, R. D. (1994). Physical properties of porcine musculature in relation to postmortem biochemical changes in muscle proteins. University of Wisconsin--Madison.
37. Pearson, A. M., & Young, R. B. (1989). Proteins of the thick filament. Muscle and meat biochemistry, 66-97 38. Apple, J. K., Minton, J. E., Parsons, K. M., & Unruh, J. A. (1993). Influence of repeated restraint and isolation stress and electrolyte administration on pituitary-adrenal secretions, electrolytes, and other blood constituents of sheep. Journal of animal science, 71(1), 71-77.
39. Babji, A. S., Froning, G. W., & Ngoka, D. A. (1982). The effect of preslaughter environmental temperature in the presence of electrolyte treatment on turkey meat quality. Poultry science, 61(12), 2385-2389.
40. Husson, F., & Pagès, J. (2003). Comparison of sensory profiles done by trained and untrained juries: methodology and results. Journal of Sensory Studies, 18(6), 453-464.
41. Lyon, B. G., Smith, D. P., Lyon, C. E., & Savage, E. M. (2004). Effects of diet and feed withdrawal on the sensory descriptive and instrumental profiles of broiler breast fillets. Poultry science, 83(2), 275-281.
42. Smith, D. P., Lyon, C. E., & Lyon, B. G. (2002). The effect of age, dietary carbohydrate source, and feed withdrawal on broiler breast fillet color. Poultry Science, 81(10), 1584-1588.
43. Schaefer, A. L., Murray, A. C., Tong, A. K. W., Jones, S. D. M., & Sather, A. P. (1993). The effect of ante mortem electrolyte therapy on animal physiology and meat quality in pigs segregating at the halothane gene. Canadian Journal of Animal Science, 73(2), 231-240









.jpg&w=3840&q=75)