Effects of prebiotic (Fermacto) in broiler chicks
Effects of prebiotic (Fermacto) in low protein diet on some blood parameters and intestinal microbiota of broiler chicks
Published: March 16, 2009
By: Mahalam Ghiyasi, Mansour Rezaei, Hadi Sayyahzadeh, Farid Firouzbakhsh, Amir Attar - Department of Animal Science. College of Animal Science and Fisheries, Mazandaran University, Sari, Iran
This study was conducted to evaluate the effects of prebiotic (Fermacto) in low protein diet on serum cholesterol and intestinal microbiota of broiler chicks. One hundred and fifty six 1-day old Ross 308 broiler chicks of both sexes were used for 42 days. The chicks were randomly allocated to 12 pens containing 13 chicks each with 3 replicates and assigned to receive one of the 4 dietary treatments of 2 levels of protein (low and high) and 2 levels of prebiotic (0 and 0.2%) in a completely randomised design with factorial arrangement. There were no significant differences in serum HDL and LDL levels among treatments. Significant differences were observed in serum cholesterol and intestinal microflora between the high protein diet without prebiotic and the low protein diet containing prebiotic (P<0.05). The results of the present experiment showed that the addition of prebiotic to broiler diets containing 90% of the NRC protein recommendation significantly affects serum cholesterol and intestinal microflora of broiler chicks (P<0.05).
Introduction
In the modern intensive poultry production, newly hatched chicks have little chance of contact with their mother; therefore, normal microflora is slow to colonize in the intestine (Fuller, 1989). This situation makes chicks likely to be affected by a small number of pathogenic bacteria due to the sterile condition of the intestine, subsequently causing food-born disease in human beings (Pivnick and Nurmi, 1982).
The use of prebiotics or fermentable sugars instead of antibiotics is going to be popular in birds in order to improve the useful microbial population of the gastrointestinal (GI) tract (Kermanshahi and Rostami, 2006). Prebiotics have been defined by Gibson and Roberfroid (1995) as indigestible food ingredients which stimulate the growth and/or activity of a selected number of bacteria in the GI tract and improve the host's health. Several studies have shown that the addition of prebiotics to the diet of broilers, layers and pigs leads to improved performance by improving gut beneficial microbiota (Spring et al., 2000; Xu et al., 2003; Pelicano et al., 2004). Prebiotics have been shown to alter GI microflora, alter the immune system, prevent colon cancer, reduce pathogen invasion including pathogens such as Salmonella enteritidis and E.coli, and reduce cholesterol and odour compounds (Cummings et al., 2001; Simmering and Blaut, 2001; Cummings and Macfarlane, 2002). The commercially available fermentation product of Aspergillus orizae, Fermacto referred to as Aspergillus meal (AM), has no live cells or spores and is proven to enhance the digestive efficiency of the gut (Harms and Miles, 1988). As Kim et al. (2003) reported, Aspergillus oryzae might act as substrates for favourable bacteria such as Lactobacillus in the intestinal microbial system that subsequently reduces Salmonella or E. coli concentrations.
High protein prices and environmental concerns have pressured the poultry industry to reduce dietary protein levels (Firman, 1997). Thus, low protein diets are of interest and important for feed additive evaluation and animal performance. AM might offer better results when the level of protein and amino acids is lower than those recommended by National Research Council (NRC, 1994) or applied in commercial flocks. Because of reports on the use of AM and low dietary protein, amino acids in broiler chicks are lacking. Therefore, the objective of the present study was to evaluate the effect of prebiotic (Fermacto) in diets containing different levels of protein on some blood parameters and the intestinal microflora population of broiler chicks.
Material and methods
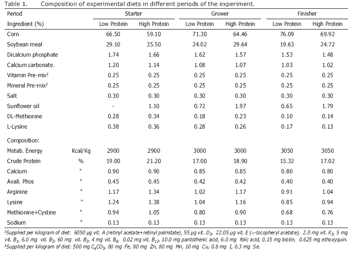
Birds and experimental diets: 156 day-old mixed Ross broiler chicks were randomly allocated to 12 groups of 13 birds each and reared for 42 days. There were four treatments (treatment 1: high protein diet without prebiotic, treatment 2: high protein diet containing prebiotic, treatment 3: low protein diet without prebiotic, treatment 4: low protein diet containing prebiotic) in this experiment. Prebiotic (Fermacto) was supplemented at the rate of 0 and 2.0 kg/ton of diets. Feed and water were provided adlibitum during the experiment. Diets were provided in 3 periods: starter (1-10 days of age); grower (10-28 days of age); and finisher (28-42 days of age). Composition of experimental diets is presented in Table 1.
Click here to enlarge the image
Sample collection

Click here to enlarge the image
Sample collection
At 10 and 28 days of age, two birds from each replicate with body weight similar to the mean pen body weight were sacrificed by cervical dislocation and the ileal samples were collected to determine the population of total intestinal aerobes and coliform. At 42 days of age, blood samples were collected from the bronchial vein of 2 chicks from each replicate to determine serum cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL). The blood was collected in a test tube to obtain serum. The collected blood samples were centrifuged at 3000×g for 10 min and the serum was decanted into aseptically treated vials and stored at -20ºC for total cholesterol, LDL, HDL. Serum cholesterol, LDL, HDL, were measured by using diagnostic kits (AGAPPE diagnostic kits) and spectrophotometer apparatus. The carcasses were immediately opened and the entire intestine removed aseptically. Approximately 1 g of the ileal content was mixed with 9 mL of produced sterile dilution blank solution (Bryant and Burkey, 1953), and homogenized for 3 min. From the initial 10-1 dilution, 10-fold sterile dilutions were subsequently made in 0.1% peptone for aerobic bacteria. The samples from the ileum were diluted to 10-6 and 1 mL was incubated on sterile plate count agar (PCA) for aerobes. Incubated plates were incubated at 370C for 2 d. Total numbers of bacterial colonies were counted at the end of each incubation period. For determination of the coliform number, eosin methylene-blue agar (EMB) was used. An amount of 0.1 mL from the initial 10-1 dilution with a micropipette, was transported to Petri dishes containing EMB, and incubated in 37°C for 2 d. After 2 d the colonies of coliform were counted via digital colony counter apparatus.
Data analysis
The data from this experiment were subjected to one-way analysis of variance as factorial arrangement 2×2 with 2 levels of protein and 2 levels of probiotic; thus there were 4 treatments and 3 replicates for each treatment. The obtained data were submitted to analysis of variance, using the General Linear Model procedure (GLM) of SAS software (SAS Institute, 2002). When significant differences were detected (P<0.05), means were compared by the Duncan's multiple range tests at 5% probability (Duncan, 1955).
Results and discussion

Results and discussion
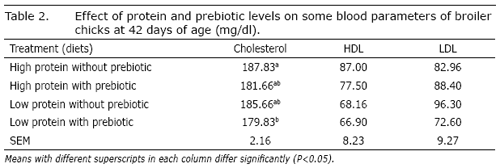
Effects of different levels of protein and prebiotics on some blood parameters of broiler chicks at 42 days of age are presented in Tables 2. Interaction of protein and prebiotic was significant for serum cholesterol level (P<0.05). Chicks fed with the low protein diet containing prebiotic (treatment 4) had lower levels of serum cholesterol than the high protein diet without prebiotic (control). Results of this study are in agreement with the findings of previous experiments (Yusrizal and Chen, 2003; Kannan et al., 2005). The hypercholesterolemia effect by Aspergillus oryzae could be related to compounds in Aspergillus oryzae that is known to inhibit cholesterol biosynthesis (Hajjaj et al., 2005). Hypercholesterolemia effect by Aspergillus oryzae can be made by monitoring a key enzyme, for example, 3-hydroxyl- 3-methylglutaryl-coenzyme A reductase in cholesterol synthesis in poultry (Lee et al., 2006). Gilliland et al. (1985) hypothesized that a decrease in cholesterol level could be due to the cholesterol assimilation by Lactobacillus. The prebiotic supplementation could have enhanced the Lactobacilli count. These researchers hypothesized that some Lactobacillus spp. are able to incorporate cholesterol into the cellular membrane of the organism, thus cholesterol assimilation by Lactobacillus in turn reduces cholesterol absorption in the system, or the coprecipitation of cholesterol with conjugated bile salt (Klaver and Van der Meer, 1993). Similar results have been reported by Mohan et al. (1996) and Kalavathy et al. (2003). A similar hypercholesterolemia effect was observed in broiler chickens supplemented with beta fructans from chicory as a source of prebiotic (Yusrizal and Chen, 2003).
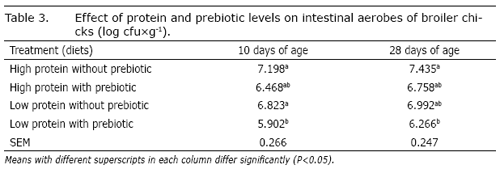
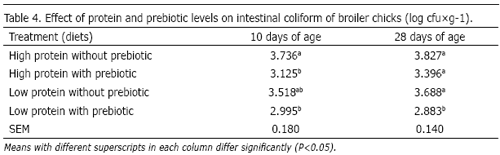
The effect of protein and prebiotic levels was also significant on the population of intestinal aerobes (P<0.05). Results are shown in Table 3. High protein diets (with and without addition of prebiotic) caused more total intestinal aerobes than the low protein diet containing prebiotic at 10 and 28 days of age. The addition of prebiotic to the low protein diet reduced total intestinal aerobes. The effect of protein and prebiotic levels on the population of intestinal coliform at 10 and 28 days of ages, was also significant (P<0.05). Results are shown in Table 4. Treatment 4 (low protein diet containing prebiotic) had the lowest and treatment 1 (high protein without prebiotic) had the highest value for this trait. It is well established that the normal microflora of the GI plays an important role in the health and well-being of poultry. Various pathogenic microbes, such as E.coli, have been implicated in reducing the growth of poultry. Possible mechanisms for this reduction of growth are toxin production, utilization of nutrients essential to the host, and supporting of microbes that synthesize vitamins or other host growth factors (Rahmani and Speer, 2005). Samli et al. (2007) reported that prebiotic increased lactic acid bacteria colonization in the ileum. Lactate is the major end product of the lactate producing bacteria, such as Lactobacillus and Bifidobacterium. An increased lactate concentration often decreases luminal pH and is a potent anti-microbial substance to several pathogenic species. Prebiotic helps to balance the intestinal microflora of poultry, resulting in a more efficient use of nutrients from the feed, more intensive processes of protein me tabolism and, subsequently, in better health (Mokslai, 2006). In some experiments, a diet containing lactobacillus cultures reduced the number of coliforms in the ceca and small intestine of broilers and turkeys (Francis et al., 1978; Jin et al., 1998).
Prebiotics have been shown to alter gastrointestinal microflora, alter the immune system, prevent colon cancer, reduce pathogen invasion including pathogens such as E. coli and reduce cholesterol and odour compounds (Cummings et al., 2001; Simmering and Blaut, 2001; Cummings and Macfarlane, 2002;). The major effects of prebiotics have been reviewed by Cummings and Macfarlane (2002) and include production of short-chain fatty acids and lactate, selective increases in bifidobacteria and lactobacilli, an increase in pathogen resistance and improved calcium and magnesium absorption.

The effect of protein and prebiotic levels was also significant on the population of intestinal aerobes (P<0.05). Results are shown in Table 3. High protein diets (with and without addition of prebiotic) caused more total intestinal aerobes than the low protein diet containing prebiotic at 10 and 28 days of age. The addition of prebiotic to the low protein diet reduced total intestinal aerobes. The effect of protein and prebiotic levels on the population of intestinal coliform at 10 and 28 days of ages, was also significant (P<0.05). Results are shown in Table 4. Treatment 4 (low protein diet containing prebiotic) had the lowest and treatment 1 (high protein without prebiotic) had the highest value for this trait. It is well established that the normal microflora of the GI plays an important role in the health and well-being of poultry. Various pathogenic microbes, such as E.coli, have been implicated in reducing the growth of poultry. Possible mechanisms for this reduction of growth are toxin production, utilization of nutrients essential to the host, and supporting of microbes that synthesize vitamins or other host growth factors (Rahmani and Speer, 2005). Samli et al. (2007) reported that prebiotic increased lactic acid bacteria colonization in the ileum. Lactate is the major end product of the lactate producing bacteria, such as Lactobacillus and Bifidobacterium. An increased lactate concentration often decreases luminal pH and is a potent anti-microbial substance to several pathogenic species. Prebiotic helps to balance the intestinal microflora of poultry, resulting in a more efficient use of nutrients from the feed, more intensive processes of protein me tabolism and, subsequently, in better health (Mokslai, 2006). In some experiments, a diet containing lactobacillus cultures reduced the number of coliforms in the ceca and small intestine of broilers and turkeys (Francis et al., 1978; Jin et al., 1998).

Prebiotics have been shown to alter gastrointestinal microflora, alter the immune system, prevent colon cancer, reduce pathogen invasion including pathogens such as E. coli and reduce cholesterol and odour compounds (Cummings et al., 2001; Simmering and Blaut, 2001; Cummings and Macfarlane, 2002;). The major effects of prebiotics have been reviewed by Cummings and Macfarlane (2002) and include production of short-chain fatty acids and lactate, selective increases in bifidobacteria and lactobacilli, an increase in pathogen resistance and improved calcium and magnesium absorption.
Conclusions
The results of the present study indicate that addition of prebiotic to low protein diets of broiler chicks significantly affects serum cholesterol and beneficial intestinal microflora. More research is needed to clarify our understanding of the optimal and marginal levels of prebiotics (Fermacto) in different species of poultry with respect to performance and health.
References
Bryant, M.P., Burkey, L.A., 1953. Cultural methods and some characteristics of some of the more numerous groups of bacteria in the bovine rumen. J. Dairy Sci. 36:205-217.
Cummings, J.H., Macfarlane, G.T., 2002. Gastrointestinal effects of prebiotics. Br. J. Nutr. 87(Suppl. 2):145-151.
Cummings, J.H., Macfarlane, G.T., Englyst, H.N., 2001. Prebiotic digestion and fermentation. Am. J. Clin. Nutr. 73(Suppl.):415-420.
Duncan, D.B., 1955. Multiple ranges and multiple F test. Biometrics 11:1-42.
Firman, J.D., 1997. Early stage turkey nutrition and ideal protein: Implications for all aspects of poultry production. In: T. P. Lyons and K. A. Jacques (eds.) Nutritional Biotechnology in the Feed and Food Industries. Nottingham University Press, Nottingham, UK, pp 57- 67.
Francis, C., Janky, D.M., Arafa, A.S., Harms, R.H., 1978. Interrelationship of lactobacillus and zinc bacitracin in diets of turkey poults. Poultry Sci. 57:1687-1689.
Fuller, R., 1989. Probiotics in man and animals. J. Appl. Bacteriol. 66:365-378.
Gibson, G.R., Roberfroid, B., 1995. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 125:1401-1412.
Gilliland, S.E., Nelson, C.R., Maxwell, C., 1985. As similation of cholesterol by Lactobacillus acidophilus. Appl. Environ. Microbiol. 49:377-381.
Hajjaj, H., Duboc, P., Fay, L.B., Zbinden, I., Mace, K., Niederberger, P., 2005. Aspergillus oryzae producs compounds inhibiting cholesterol biosynthesis downstream of dihydrolanosterol. FEMS Microbiol. Lett. 242:155-159.
Harms, R.H., Miles, R.D., 1988. Research note: Influence of Fermacto on the performance of laying hens when fed with different levels of methionine. Poultry Sci. 67:842-844.
Jin, L.Z., Ho, Y.W., Abdullah, N., Jalaludine, S., 1998. Growth performance, intestinal microbial population, and serum cholesterol of broilers fed diets containing lactobacillus cultures. Poultry Sci. 77:1259-1265.
Kalavathy, R., Abdullah, N., Jalaludin, S., Ho, Y.W., 2003. Effect of Lactobacillus cultures on growth performance, abdominal fat deposition, serum lipids and weight of organs of broiler chickens. Brit. Poultry Sci. 44:139-144.
Kannan, P., Karunakaran, R., Balakrishnan, V., Prabhakar, T.G., 2005. Influence of prebiotics supplementation on lipid profile of broilers. Int. J. Poultry Sci. 4:994-997.
Kermanshahi, H., Rostami, H., 2006. Influence of supplemental dried whey on broiler performance and cecal flora. Int. J. Poultry Sci. 5:538-543.
Kim, S.H., Park, S.Y., Yu, D.J., Lee, S.L., Ryu, K.S., Lee, D.G., 2003. Effects of feeding Aspergillus oryzae ferments on performance, intestinal microbiota, blood serum components and enviromental factors in broiler. Korean J. Poultry Sci. 30:151-159.
Klaver, F.A.M., Van der Meer, R., 1993. the assumed assimilation of cholesterol by lactobacilli and biofidobacterium biofidum is due to their bile saltdeconjugating activity. Appl. Environ. Microbiol. 59:1120-1124.
Lee, K.,. Lee, S.K., Lee, B.D., 2006. Aspergillus oryzae as probiotic in poultry-a review. Int. J. Poultry Sci. 5:1-3.
Mohan, B., Kadirvel, R., Natarajan, A., Bhaskaran, M., 1996. Effect of probiotic supplementation on growth, nitrogen utilization and serum cholesterol in broilers. Brit. Poultry Sci. 37:395-401.
Mokslai, Ž.Ü., 2006. Influence of a prebiotic feed additive on some biochemical indices of blood and intestinal microbiota of broiler chickens. J. Nutr. 4:57-62.
National Research Council., 1994. Nutrient Requirements of Poultry. 9th rev. ed. National Academy Press, Washington, D.C., USA.
Pelicano, E.R.L., De Souza, P.A., De Souza, H.B.A., Leonel, F.R, Zeola, N.M.B.L., Boiago, N.M.B.L., 2004. Productive traits of broiler chickens fed diets containing different growth promoters. Page 18 in Proc. Int. Asian Tech. Conf., Santos, Sao Paulo, Brazil.
Pivnick, H., Nurmi, E., 1982. The Nurmi concepts and its role in the control of Salmonella in poultry. In: I.R. Davis (ed.) Development in food microbiology. Applied Science Publishers, Essex, UK, pp 41-70.
Rahmani, H.R., Speer, W., 2005. Natural additives influence the performance and humoral immunity of broilers. Int. J. Poultry Sci. 4:713-717.
Samli, H.E, Senkoylu, N., Koc, F., Kanter, M., Agma, A., 2007. Effects of Enterococcus faecium and dried whey on broiler performance, gut histomorphology and intestinal microbiota. Arch. Anim. Nutr. 61:42-49.
SAS, 2002. User's Guide Statistics. Version 8.1. SAS Institute Inc., Cary, NC, USA.
Simmering, R., Blaut, M., 2001. Pro- and Prebiotics the tasty guardian angles? Appl. Microbiol. Biotechnol. 55:19-28.
Spring, P., Wenk, C., Dawson, K.A., Newman, K.E., 2000. The effects of dietary mannan oligosaccharides on cecal parameters and the concentrations of enteric bacteria in the caeca of Salmonella - challenged broiler chicks. Poultry Sci.79:205-211.
Xu, Z.R., Hu, C.H., Xia, M.S., Zhan, X.A, Wang, M.Q., 2003. Effects of dietary fructo oligosaccharide on digestive enzyme activities, intestinal microbiota and morphology of male broilers. J. Anim Sci. 82:1030-1036.
Yusrizal, Y., Chen, T.C., 2003. Effect of adding chicory fructans in feed on fecal and intestinal microflora and excreta volatile ammonia. Int. J. Poultry Sci. 2:188-194.
Related topics:
Authors:
Javaneh khorasan
Recommend
Comment
Share
Recommend
Reply
Guybro Chemical
18 de marzo de 2009
A good effort to evaluate benefit of Prebiotic in low protein diet. More comprehensive studies may popularize Prebiotic to replace AGP in due course of time.
Recommend
Reply

Would you like to discuss another topic? Create a new post to engage with experts in the community.










.jpg&w=3840&q=75)