Introduction
Campylobacter infections pose a serious public health problem; the incidence of campylobacteriosis has progressively increased in developed countries, and the pathogen is now considered the leading cause of bacterial gastroenteritis throughout the world (Humphrey et al. 2007; FAO⁄WHO, 2009). Thermophilic Campylobacter jejuni and Campylobacter coli are the most frequently isolated species in foodborne zoonoses in humans (EFSA Journal, 2011). Campylobacter can establish itself as a subclinical infection in humans, but frequently causes a range of clinical symptoms varying from self-limited, mild diarrhea to severe inflammatory bloody diarrhoea. Occasionally, acute or long-term and potentially serious complications occur such as septicaemia, irritable bowel syndrome, reactive arthritis or autoimmune neuropathies (Guillain-Barre´ and Miller Fisher Syndrome) (Godschalk et al. 2004; Leonard et al. 2004; Takahashi et al. 2005; Humphrey et al. 2007). Large outbreaks are uncommon, and the vast majority of human campylobacteriosis cases are sporadic; they most likely result from handling or consumption of raw or undercooked contaminated meat products. Other foodstuffs, untreated drinking water and milk have also been associated with the illness, but poultry products are considered the major source of infection (Pebody et al. 1997; Altekruse et al. 1999; Pires et al. 2010).
Bacteriological culture of Campylobacter spp. can be a challenge, owing to the fragility of these organisms. The use of a selective medium is recommended for the recovery from stool and faeces; for samples with low bacterial numbers, filtration or enrichment steps are typically added to improve recovery (Hu and Kuo 2011). Direct plating on selective agar media is common practice for Campylobacter isolation from several matrices (drinking water, environmental (dust) or intestinal samples), but an ideal single method for the entire range of samples requiring testing has not been developed (Baylis et al. 2000; Engberg et al. 2000; Musgrove et al. 2001; Commission Decision 2007 ⁄ 516 ⁄ EC). In 2006, the International Organization for Standardization (ISO) standard method for detection of Campylobacter spp. in food recommended enrichment using Bolton broth, followed by culture on selective modified charcoal cefoperazone desoxycholate agar (mCCDA) and one other alternative agar plate (ISO, 2006).
For our study, which covered various matrices, we compared the results of traditional culturing methods and a real-time quantitative PCR assay, in an attempt to combine optimal sensitivity with short isolation and confirmation time. We evaluated three different procedures for Campylobacter isolation: direct plating on selective media [mCCDA or Campyfood Agar (CFA)], four combinations of enrichment and plating media (Bolton or Preston enrichment, combined with mCCDA or CFA plates) and molecular detection by real-time PCR (qPCR). The evaluation was performed on naturally contaminated broiler faeces, neck skin and poultry meat samples.
Materials and methods
Samples
A total of 114 chicken samples were tested from April 2010 to February 2011 consisting of neck skin (n = 38), breast meat (n = 38) and faecal samples (n = 38). From individual batches of birds, intestines (n = 380, ten homogenized caecum contents per sample), neck skin (n = 38) and packaged breast meat specimens (n = 28) were obtained at the slaughterhouse after evisceration (caecum), immediately after chilling (neck skin) and at the end of the processing line (meat). In addition, independent breast meat packages (n = 10) were sampled at retail. All samples were kept refrigerated during transport to the laboratory, and culture was performed immediately after reception. In addition, 300 mg of each fresh sample was stored at )40_C for subsequent DNA extraction and qPCR.
Method 1: direct plating onto selective medium (mCCDA and CFA)
For direct plating of stool samples, a swab was dipped into the sample and streaked onto selective plates. For neck skin and meat samples, a surface of approximately 5 cm2 was swabbed. All swabs were directly streaked onto Campylobacter blood-free selective medium (mCCDA, modified charcoal cefoperazone desoxycholate agar, CM739; Oxoid, Basingstoke, UK) and onto ready-to-use, chromogenic-like CFA plates (Campyfood agar; Ref 43471, bioMe´rieux, Marcy l’Etoile, France). Following incubation at 42_C for 48 h under microaerobic conditions (Genbag microaerobic atmosphere generator, Ref 45532, bioMe´rieux), the plates were examined. Up to five colonies with Campylobacter-typical morphology (according to the manufacturer’s instruction) were cultured onto blood agar plates (bioMe´rieux) at 37_C for 48 h in a microaerobic atmosphere for further identification using conventional PCR. If more than one colony morphology was observed, representative colonies of these were picked. A sample was considered positive if at least one colony was confirmed by PCR.
Method 2: ISO 10272:2006-1 using enrichment with Bolton broth
The recommended ISO 10272:2006-1 protocol included enrichment in Bolton broth (CM0983; Oxoid) supplemented with antibiotic supplement (SR0183) and 5% lysed horse blood (SR0048) (both from Oxoid). One gram of neck skin was aseptically transferred to a 10-ml sterile screwcap bottle, and 9 ml Bolton broth was added. Meat samples (25 g taken from the surface) were transferred to sterile stomacher bags with filter and pouch and mixed with 225 ml Bolton broth, while 10 g of fresh faeces was mixed in stomacher bags with 90 ml Bolton broth. These were incubated with a Genbox atmosphere generator. Enrichment was performed for 4–6 h at 37_C followed by 48 h at 42_C, after which 200 ll was cultured for 48 h on the two selective agar plates (mCCDA and CFA) as described above.
Method 3: enrichment method using Preston broth
The third tested procedure was based on a previous recommendation described in ISO 10272:1995-1 (ISO, 1995, now withdrawn) and included enrichment using Preston broth (Nutrient broth No. 2, CM0067; Oxoid) that was prepared according to the manufacturer’s instructions and supplemented with 5% lysed horse blood (SR0048) and antibiotic (SR0204 and SR0232E; Oxoid). The enrichment step with Preston broth was performed at 42_C for 48 h according to Corry et al. 1995 (though ISO 10272:1995-1 recommended 18 h), and all further steps were performed as described in Method 2.
Identification of suspected Campylobacter colonies
Suspected Campylobacter colonies were picked and subcultured onto blood agar plates (bioMe´rieux) by microaerobic incubation at 37_C for 48 h. DNA was liberated by boiling a colony, suspended in 600 ll of sterile double distilled water, for 10 min.
Conventional multiplex PCR was used for simultaneous identification of the genus Campylobacter and the differentiation between Camp. jejuni and Camp. coli. All primers were designed by Oligo 6.0 software (Molecular Biology Insights, Cascade, CO, USA). For genus identification, a primer set specific for the 16S rRNA gene of all Campylobacter spp. was designed based on 79 sequences (14 Camp. jejuni, 13 Camp. coli, 47 Campylobacter spp. and five from other genera). Primer 16s1 (5´-GGATGACACTTTTCGGAGC) combined with degenerated primer 16s2 (5´-TTDGYATTYCSGCTTCGAGT) produced a 1039-bp amplicon. Their specificity was verified using 30 strains of Campylobacter spp. that had been speciated based on biochemical characterization as well as PCR identification (Mateo et al. 2005). For identification of Camp. coli, species-specific primers targeting ceuE (enterochelin uptake periplasmic-binding protein gene) were designed, based on 30 different Camp. jejuni and Camp. coli ceuE sequences. The primers COL1 (5´-ACTTTCCATGCCCTAAGAC) and COL2 (5´-TCCACCTATACTAGGCTTGTC) produced a 102-bp amplicon for Camp. Coli only. These primers were verified using 24 Camp. Jejuni and Camp. coli strains that had been unambiguously speciated, while 20 strains of other Campylobacter species did not produce an amplicon. The strains used for verification included Camp. jejuni ATCC 33560 and Camp. coli CRL C 2Æ2, (2007) that were obtained from the EU Reference Laboratory of Antimicrobial Resistance (Technical University of Denmark, Lyngby, Denmark). For the identification of Camp. jejuni, the hipO gene (hippurate hydrolase) was chosen, and for primer selection, 40 different sequences of Camp. jejuni were compared. A 130-bp amplicon was obtained using JEJ1 (5´-CTCCTATGCTTACAACTGCTG) and JEJ2 (5´-GGTGGTCATGGAAGTGCT) whose specificity was verified as above. Furthermore, positive controls were included using DNA from Camp. jejuni strain ATCC 33560 and Camp. coli strain CRL C 2.2, and a negative control contained all reagents except DNA. PCR amplification was performed in 20 ll containing 1Æ8 ll of lysed cell supernatant, 10 ll of a PCR master mix (kit Qiagen Multiplex PCR; Hilden, Germany) and 0Æ19 lmol l)1 of each primer (Invitrogen, Life Technologies, Paisley, UK). The amplification was performed in a Thermal Cycler (C1000; Bio-Rad Laboratories, Hercules, CA, USA) with denaturation for 15 min at 95_C, 35 cycles with 30 s at 95_C, 90 s at 56_C and 1 min at 72_C and a final 10-min extension at 72_C. Amplicons were detected by gel electrophoresis using 2% agarose gels containing 10 mg ml)1 SYBR green stain (Invitrogen, Life Technologies) for 40 min at 400 mA. A DNA molecular weight marker (100-bp low ladder; Biotools, B&M Labs, Madrid, Spain) was included for reference. Bands were visualized under UV light, and gel images were taken with a UV Bio-Rad Molecular Imager (Bio-Rad).
A sample was considered confirmed if the genusspecific amplicon as well as either a Camp. coli or a Campy. jejuni-specific amplicon was obtained from a colony.
Method 4: molecular detection (multiplex real-time PCR)
DNA was extracted from 300 mg of neck skin or meat using the QIAamp DNA Mini Kit 50 (Qiagen) and from 300 mg stool using the QIAamp DNA Stool Mini Kit 50 (Qiagen). Extracted DNA (eluated in 130 ll) was subjected to an in-house multiplex real-time PCR assay using the ceuE and hipO amplification primers as mentioned above, to detect and differentiate both species in a single reaction. Fluorophore-linked probes were added for detection of the amplicons: Camp. Jejunispecific hipO amplicon was detected using probe HEX- 5´-AGATCCTATTTATGCTGCTTCTTTRC-BHQ, and the Camp. coli-specific cueE amplicon was detected with probe FAM-5?-ATAAAGTTGCAGGAGTTCCAGCTAAABHQ. The specificity of these hydrolysis probes was confirmed using the set of 24 Camp. jejuni and Camp. coli, as well as 20 strains of other Campylobacter species, described above. All reactions were carried out in triplicate with inclusion of a negative template control as well as positive controls. For generation of a standard curve, 1 ng DNA of Camp. jejuni ATCC 33560 was mixed with 1 ng DNA of Camp. coli CRL C 2.2, and ten-fold serial dilutions were produced up to 10)4 (range, 5Æ649 · 105– 5Æ649 · 101 DNA copies). When tested, a 10)5 dilution of this standard mixture frequently remained negative. All standard dilutions and samples were performed in triplicate. The simultaneous detection and quantification of Camp. jejuni and Camp. coli allowed detection of contamination by more than one Campylobacter species.
The multiplex PCR was performed using an iCycler thermal cycler (Bio-Rad Laboratories). Reactions (final volume 25 ll) contained 5 ll of template DNA, 12Æ5 ll of QuantiTect Multiplex PCR No ROX Mastermix (QIAGEN), 0Æ4 lmol l)1 of each amplification primer and 0Æ25 lmol l)1 of each probe. The thermal cycle protocol included initial denaturation at 95_C for 15 min, followed by 40 cycles (94_C for 1 min, 56_C for 1 min) and a final extension at 72_C for 10 min. Fluorescence of FAM and HEX was measured at their respective wavelengths during the annealing step of each cycle. An internal amplification control was included in the form of a construct of 111 bp of foreign sequence (derived from Oncorhynchus mykiss viperin NCBI accession number: NM_001124253.1) flanked by the Camp. jejuni-specific hipO primers.
Data analysis
Data were analysed using spss (19.0 IBM, Chicago, Il, USA). Significance of differences (P < 0Æ05) between proportions of positive samples obtained with the different protocols was assessed using chi-squared and Fisher’s exact test depending on sample size. Quantitative results of qPCR were transformed to base-10 log values. Correlations between Camp. coli and Camp. jejuni results were evaluated using Spearman’s rank correlation coefficient.
Results
The results of the four detection methods, regardless of the Campylobacter species detected, are summarized in Table 1. Irrespective of the sample type, between 22Æ8% and 60Æ5% were found positive by culture-dependent methods 1–3, depending on the method, while qPCR detected 98Æ2% of positive samples. Direct plating was more sensitive than the two enrichment-dependent methods, and this difference was highly significant when compared with Bolton enrichment (P < 0Æ001; there was no significant difference between direct plating and Preston enrichment for all samples combined). When enrichment was included, Preston broth performed better than Bolton, and this difference was highly significant (P < 0Æ001). The ISO standard protocol performed worst for all three sample types. However, the alternative methods performed differently depending on the type of sample matrix. For faeces and neck skin samples, direct plating resulted in the highest numbers of positives, although the difference between direct plating and Preston enrichment was not statistically significant for neck skin samples. In contrast, for meat samples, enrichment with Preston broth was superior to the other two methods (P = 0Æ001, between Preston and Bolton enrichment; P < 0Æ001, between Preston enrichment and direct plating).
Table 1 also summarizes the number of samples found positive with any combination of enrichment broth and culture plates. Although this resulted in the same total of 69 positive samples as direct plating did, the number of positive faecal samples obtained with enrichment was lower, while that of positive meat samples was higher than what was obtained by direct plating. The poor performance of enrichment of stool samples was most likely due to competing intestinal microbiota, while the Preston enrichment improved detection of Campylobacter in meat, probably reflecting the lower initial bacterial load of these samples. Of the 69 samples that were positive by enrichment with at least one of the two tested broths, only 21 (30%) tested positive using both broths. Quantitative PCR was performed on all samples, and this was the most sensitive method tested. As expected, all samples found positive by culture were also positive by qPCR. The results obtained with qPCR were interpreted to reflect a theoretical maximum of positive samples, and they could be an overestimate; culture-negative but qPCR-positive samples might be due to detection of noncultivable and dead cells. Taking the qPCR as the theoretical maximum (100%) of detection, the results of the other methods were expressed as a fraction of this to visualize their respective performance (Fig. 1).
Table 1: Results obtained from 114 samples of neck skin (n = 38), faeces (n = 38) and chicken meat (n = 38) using direct plating, enrichment protocols (Bolton, Preston or both broths) and molecular detection of Campylobacter
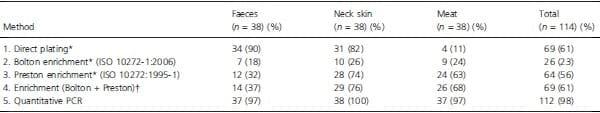
*Considered positive when at least one confirmed colony was present on either selective agar [mCCDA or Campyfood agar (CFA)].
_Considered positive when at least one enrichment broth (Bolton or Preston) resulted in a confirmed colony on either selective agar (mCCDA or CFA).
All tested culture-dependent methods included, as a final step, incubation on both mCCDA and CFA agar plates, and performance of these two selective media is compared in Table 2. Direct plating on CFA produced more positives than direct plating on mCCDA, for all matrices except for meat; for that matrix, direct plating was not as sensitive as enrichment, and there was no difference between the two agars tested. The difference between direct plating of faecal samples onto CFA or mCCDA was not statistically significant, but these selective media only moderately agreed for faecal samples (Kappa value, 0Æ350) so that samples detected positive by one could be missed by the other. Following enrichment in Bolton broth, CFA plates recovered more positives than mCCDA plates, for all matrices. Only for meat samples did enrichment in Preston, combined with selective culture on mCCDA, perform better than the other methods (Table 2). Nevertheless, Method 3 (Bolton enrichment) followed by CFA plating identified two meat samples as positive that were missed by all other culture methods.
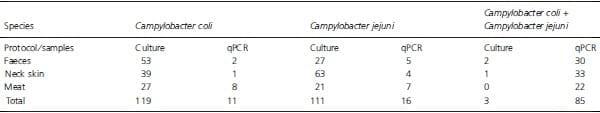
These data suggest that the culture-dependent speciation of single colonies might have underestimated the true diversity of the bacterial population, because the majority of samples (85 of 112) turned out to contain DNA for both Camp. jejuni and Camp. coli.
Table 2: Results obtained from neck skin, faeces and chicken meat samples with the two types of selective agar plates (mCCDA or CFA), with or without enrichment (Bolton or Preston)
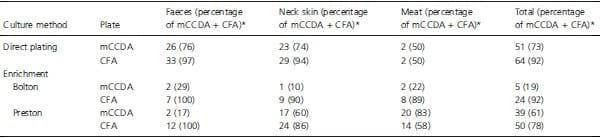
*For absolute values of the readings taken on mCCDA + CFA plates combined, see Table 1.
Table 3: Obtained Campylobacter species from 114 samples of neck skin, faeces and chicken meat using all tested culture protocols (direct plating, Bolton and Preston enrichment) as well as molecular detection
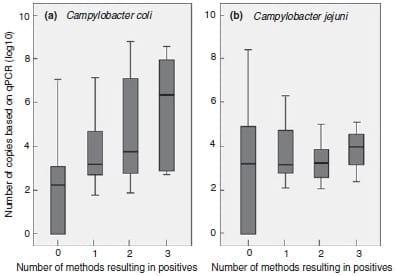
The proportion of positive samples for each species detected by qPCR did not differ significantly; the obtained quantitative data for all samples are summarized in Table 4. The highest amount of Campylobacter DNA was detected in faecal samples, for both species. Higher mean and median values were observed for Camp. Jejuni than for Camp. coli, for all three types of samples. For those samples found positive of both species, the quantity of one species is weakly correlated (Spearman’s rho = 0Æ410) with that of the other. In the box-and-whiskers plot of Fig. 2, the base-10 log values were related to the number of culture-dependent protocols that were able to detect each species. Comparing the results for the two species shows that, for Camp. coli, more methods detect this species as the bacterial load per sample increased. This was not observed for Camp. jejuni.
Table 4: Quantitative data obtained by qPCR.
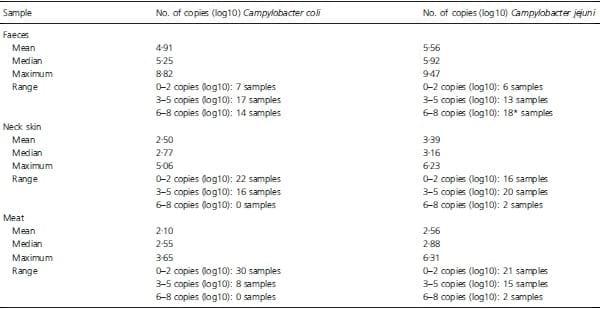
*One additional sample contained more than 9 log(10) copies of Camp. jejuni
Figure 2: Box-and-whisker plot relating the quantitative base 10 log value from qPCR for Campylobacter coli (a) and Campylobacter jejuni (b) to the number of methods (direct plating, Bolton enrichment ISO 10272-1:2006 and Preston enrichment ISO 10272:1995-1) found positive.
Discussion
Culture-dependent detection of Campylobacter from food sources has improved much since the introduction of selective agar media and enrichment broths. Selective media contain antibiotics to suppress the growth of competing organisms. Typically, cefoperazone, cycloheximide, trimethoprim, rifampicin, vancomycin or polymyxin B are used in various combinations. The most common culture methods make use of blood-based, antibioticcontaining media, such as Skirrow’s, Butzler’s and Campy-BAP media (Baylis et al. 2000; Granato et al. 2010). The blood-free, charcoal-containing selective medium mCCDA is typically used for cultural recovery from stool specimens (Aspinall et al. 1993; Corry et al. 1995; Engberg et al. 2000).
Broth enrichment is essential when low numbers of (damaged) Campylobacter are present in the sample, and the most commonly used enrichment media are Preston, Bolton or Campylobacter enrichment broth. These differences in methodology can potentially skew results when the complete food chain is being investigated. Bolton broth is currently the medium recommended by the US Food and Drug Administration, the International Standard Organization (International Organization for Standardization (ISO) (2006) and the Nordic Committee of Food Analysis (Habib et al. 2011). It is realized that the enrichment step has to compromise between selectivity and the inhibition of competitor organisms, together with the recovery and growth of the target organism to detectable levels (Baylis et al. 2000).
When analysing large numbers of samples, the workload should be minimized, and avoidance of duplication of selective agar, or omission of an enrichment step, might be an attractive choice, even accepting a possible consequential lesser sensitivity. In the present study, the fast, simple and cheap method of direct plating was shown to yield the best isolation efficiency for detection of Campylobacter in faeces and neck skin samples. For these matrices, enrichment hampered effective detection, especially for faecal samples. Although the difference between mCCDA and CFA was not statistically significant, we recommend the latter type of selective plates, because colony identification is easier on CFA plates than on mCCDA. According to Kiess et al. (2010), direct plating significantly increased isolation of Campylobacter from litter samples when compared with Campylobacter enrichment broth (CEB). Musgrove et al. (2001) observed a decrease of 36Æ7% in the detection of Campylobacter spp. in caecal samples caused by enrichment when compared with the direct plating procedure. Omitting the enrichment could reduce sensitivity for neck skin samples, as suggested by our findings: four samples were negative by direct plating that showed up positive after enrichment with Preston. However, the reverse was true as well, and in total, more positive neck skin samples were detected by direct plating than by enrichment.
Typically, Campylobacter is present on food at much lower levels than in faecal samples, so that for meat samples, an enrichment step is necessary. Food samples typically contain injured and dead cells as a result of exposure to heating, chilling, freezing or others detrimental conditions related to food processing and storage (Rosenquist et al. 2006). In an early study, Bolton broth was found to be the best compromise between inhibition of competing microflora and growth of Campylobacter, when compared with Preston or CEB (Baylis et al. 2000). However, our results identified that for meat samples, Preston broth and subsequent plating on mCCDA resulted in a significantly higher recovery of Campylobacter than the current ISO 10272:2006-1 (P = 0Æ001). To increase sensitivity, after Preston enrichment of meat samples, both mCCDA and CFA plates should be used, as the concordance of the two selective media was low (Kappa value, 0Æ273).
On the basis of our results for all the matrices tested here, Preston enrichment (which contains rifampicin and polymixin) would be better for Campylobacter isolation than Bolton broth (containing cefoperazone). Recovery of Campylobacter using Bolton broth is influenced by the choice of the subsequent plating agar, and our data produced better results for Bolton combined with CFA than with mCCDA. According to Jasson et al. (2009), Bolton broth allowed growth of extended spectrum beta-lactamase Escherichia coli present in poultry meat and these bacteria can mask the growth of Campylobacter, leading to false-negative results. Overgrowth by Pseudomonas spp., which are also frequently present in food stuffs (Baylis et al. 2000), is another problem, attributable to the absence of polymixin and rifampicin in Bolton broth. A revision of ISO 10272 Part 1 and Part 2:2006 is in progress by the EURL (The European Union Reference Laboratory for Campylobacter. National Veterinary Institute, SVA, Uppsala, Sweden). Proficiency tests showed that Preston broth was superior to Bolton broth for samples with high background flora of multiresistant E. coli, but for samples with low numbers of Campylobacter or samples containing Campylobacter lari, Bolton broth seemed to be a better alternative (Olsson Engvall et al. 2011).
A culture-independent approach based on DNA amplification (qPCR) has several advantages over classical bacteriology for Campylobacter detection, notably a faster performance combined with a lower detection limit. Moreover, PCR will detect viable but not cultivable cells, for which it is unknown whether they provide a risk for consumers (Nogva et al. 2000; Humphrey et al. 2007). Real-time PCR yields highly sensitive and specific results while avoiding manipulation of PCR products after amplification, thereby reducing the risk of cross-contamination; it can be used for rapid quantitative screening of samples (Debretsion et al. 2007; Botteldoorn et al. 2008; Melero et al. 2011). However, phenotypic expression of certain properties cannot be tested, and, without cultures, additional information such as subtyping or antimicrobial resistance testing cannot be obtained. A potential disadvantage of PCR-dependent techniques is that they may overestimate the number of pathogens present in a matrix, as dead cells will also be detected. Therefore, qPCR results can be considered the theoretical maximum of detectable micro-organisms, accepting that this may be an overestimate as molecular detection also reports the presence of dead cells. We used qPCR-positive results as a maximum value to correlate culture-dependent results. Interestingly, for Camp. coli, we observed a relationship between the qPCR quantitative values and the number of protocols at which each sample yielded positive, but this was not the case for Camp. jejuni. Further studies are needed to confirm this result and to investigate the reason for this difference.
In summary, direct plating on CFA selective agar resulted in optimal Campylobacter isolation for highly contaminated samples such as faeces and neck skin, while enrichment in Preston broth offers reliable recovery from matrices containing low levels of (damaged) organisms. The internationally recommended ISO method is not the best choice for detection of Campylobacter spp. in the food chain.
Acknowledgements
This study is part of Project AGL2009-07550 (Campylobacter: molecular detection and characterization. Analysis on virulence, genotype and possible intervention strategies) funded by the Ministry of Science and Innovation, Government of Spain. This work was partially supported by contracts with the Ministry of Environment, Rural and Marine Affairs and the Autonomous Community of Madrid and grant S2009 ⁄ AGR-1489 from the Community of Madrid, Spain. The PhD grant of María Ugarte Ruiz was funded by the Spanish Ministry of Education, Culture and Sports. The authors wish to thank all Foodborne Zoonoses and Antibiotic Resistance Unit staff for their excellent work.
This article was originally published in Journal of Applied Microbiology 2012 Jul;113(1):200-8. doi: 10.1111/j.1365-2672.2012.05323.
References
1. Altekruse, S.F., Stern, N.J., Fields, P.I. and Swerdlow, D.L. (1999) Campylobacter jejuni – An emerging foodborne pathogen. Emerg Infect Dis 5, 28–35.
2. Aspinall, S.T., Wareing, D.R., Hayward, P.G. and Hutchinson, D.N. (1993) Selective medium for thermophilic Campylobacters including Campylobacter upsaliensis. J Clin Pathol 46, 829–831.
3. Baylis, C.L., MacPhee, S., Martin, K.W., Humphrey, T.J. and Betts, R.P. (2000) Comparison of three enrichment media for the isolation of Campylobacter spp. from foods. J Appl Microbiol 89, 884–891.
4. Botteldoorn, N., Van Coillie, E., Piessens, V., Rasschaert, G., Debruyne, L., Heyndrickx, M., Herman, L. and Messens, W. (2008) Quantification of Campylobacter spp. in chicken carcass rinse by real-time PCR. J Appl Microbiol 105, 1909–1918.
5. Commission Decision 2007 ⁄ 516 ⁄ EC. (2007). Concerning a financial contribution from the Community towards a survey on the prevalence and antimicrobial resistance of Campylobacter spp. in broiler flocks and on the prevalence of Campylobacter spp. and Salmonella spp. in broiler carcasses to be carried out in the Member States.
6. Corry, J.E.L., Post, D.E., Colin, P. and Laisney, M.J. (1995) Culture media for the isolation of Campylobacters. Int J Food Microbiol 26, 43–76.
7. Debretsion, A., Habtemariam, T., Wilson, S., Nganwa, D. and Yehualaeshet, T. (2007) Real-time PCR assay for rapid detection and quantification of Campylobacter jejuni on chicken rinses from poultry processing plant. Mol Cell Probes 21, 177–181.
8. Engberg, J., On, S.L., Harrington, C.S. and Gerner-Smidt, P. (2000) Prevalence of Campylobacter, Arcobacter, Helicobacter, and Sutterella spp. in human fecal samples as estimated by a reevaluation of isolation methods for Campylobacters. J Clin Microbiol 38, 286–291.
9. European Food Safety Authority, European Centre for Disease Prevention and Control. (2011) The European Union Summary Report on Trends and Sources of Zoonoses, Zoonotic Agents and Food-borne Outbreaks in 2009. EFSA Journal 9, 2090 doi: 10.2903/j.efsa.2011.2090.
10. FAO ⁄WHO [Food and Agriculture Organization of the United Nations ⁄ World Health Organization]. (2009) Risk assessment of Campylobacter spp. In broiler chickens: interpretative summary. Microbiological Risk Assessment Series No. 11. Geneva. pp. 35.
11. Godschalk, P.C.R., Heikema, A.P., Gilbert, M., Komagamine, T., Ang, C.W., Glerum, J., Brochu, D., Li, J.J. et al. (2004) The crucial role of Campylobacter jejuni genes in anti-ganglioside antibody induction in Guillain-Barre syndrome. Journal of Clinical Investigation 114, 1659–1665.
12. Granato, P.A., Chen, L., Holiday, I., Rawling, R.A., Novak- Weekley, S.M., Quinlan, T. and Musser, K.A. (2010 Comparison of Premier CAMPY Enzyme Immunoassay (EIA), ProSpecT Campylobacter EIA, and ImmunoCard STAT! CAMPY Tests with Culture for Laboratory Diagnosis of Campylobacter Enteric Infections. J Clin Microbiol 48, 4022–4027.
13. Habib, I., Uyttendaele, M. and De Zutter, L. (2011) Evaluation of ISO 10272:2006 standard versus alternative enrichment and plating combinations for enumeration and detection of Campylobacter in chicken meat. Food Microbiol 28, 1117–1123.
14. Hu, T.L. and Kuo, P.C. (2011) Isolation of Campylobacter spp. in surface waters of Taiwan. J Microbiol Immunol Infect 44, 15–20.
15. Humphrey, T., O’Brien, S. and Madsen, M. (2007) Campylobacters as zoonotic pathogens: a food production perspective. Int J Food Microbiol 117, 237–257.
16. International Organization for Standardization (ISO). (1995). ISO 10272-1 Microbiology of food and animal feeding stuffs- horizontal method for detection and enumeration of Campylobacter spp.- Part 1: detection method. ISO 10272-1: 1995.
17. International Organization for Standardization (ISO). (2006). ISO 10272-1 Microbiology of food and animal feeding stuffs- horizontal method for detection and enumeration of Campylobacter spp.- Part 1: detection method. ISO 10272-1: 2006.
18. Jasson, V., Sampers, I., Botteldoorn, N., Lopez-Galvez, F., Baert, L., Denayer, S., Rajkovic, A., Habib, I. et al. (2009) Characterization of Escherichia coli from raw poultry in Belgium and impact on the detection of Campylobacter jejuni using Bolton broth. Int J Food Microbiol 135, 248–253.
19. Kiess, A.S., Parker, H.M. and McDaniel, C.D. (2010) Evaluation of different selective media and culturing techniques for the quantification of Campylobacter ssp from broiler litter. Poult Sci 89, 1755–1762.
20. Leonard, E.E., Tompkins, L.S., Falkow, S. and Nachamkin, I. (2004) Comparison of Campylobacter jejuni isolates implicated in Guillain-Barre syndrome and strains that cause enteritis by a DNA microarray. Infect Immun 72, 1199–1203.
21. Mateo, E., Ca´rcamo, J., Urquijo, M., Perales, I. and Ferna´ndez- Astorga, A. (2005) Evaluation of a PCR assay for the detection and identification of Campylobacter jejuni and Campylobacter coli in retail poultry products. Res Microbiol 156, 568–574.
22. Melero, B., Cocolin, L., Rantsiou, K., Jaime, I. and Rovira, J. (2011) Comparison between conventional and qPCR methods for enumerating Campylobacter jejuni in a poultry processing plant. Food Microbiol 28, 1353–1358.
23. Musgrove, M.T., Berrang, M.E., Byrd, J.A., Stern, N.J. and Cox, N.A. (2001) Detection of Campylobacter spp. in ceca and crops with and without enrichment. Poult Sci 80, 825–828.
24. Nogva, H.K., Bergh, A., Holck, A. and Rudi, K. (2000) Application of the 5 ‘-nuclease PCR assay in evaluation and development of methods for quantitative detection of Campylobacter jejuni. Appl Environ Microbiol 66, 4029– 4036.
25. Olsson Engvall, E., Lahti, E., Lindgren, G., Nyman, A., Harborn, B., Pudas, N., Svensson, L. and Hansson, I. (2011) The European Union Reference Laboratory for Campylobacter is hosted by the National Veterinary Institute, SVA, Uppsala. EuroReference J. Reference 5, 6–9. Available at http://www.anses.fr/euroreference/numero5/index.htm Pebody, R.G., Ryan, M.J. and Wall, P.G. (1997) Outbreaks of Campylobacter infection: rare events for a common pathogen. Commun Dis Rep CDR Rev 7, R33–R37.
26. Pires, S.M., Vigre, H., Makela, P. and Hald, T. (2010) Using outbreak data for source attribution of human Salmonellosis and Campylobacteriosis in Europe. Foodborne Pathogens and Disease 7, 1351–1361.
27. Rosenquist, H., Sommer, H.M., Nielsen, N.L. and Christensen, B.B. (2006) The effect of slaughter operations on the contamination of chicken carcasses with thermotolerant Campylobacter. Int J Food Microbiol 108, 226–232.
28. Takahashi, M., Koga, M., Yokoyama, K. and Yuki, N. (2005) Epidemiology of Campylobacter jejuni isolated from patients with Guillain-Barre and Fisher syndromes in Japan. J Clin Microbiol 43, 335–339.
 *Considered positive when at least one confirmed colony was present on either selective agar [mCCDA or Campyfood agar (CFA)].
*Considered positive when at least one confirmed colony was present on either selective agar [mCCDA or Campyfood agar (CFA)].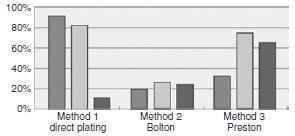
 *For absolute values of the readings taken on mCCDA + CFA plates combined, see Table 1.
*For absolute values of the readings taken on mCCDA + CFA plates combined, see Table 1.
 *One additional sample contained more than 9 log(10) copies of Camp. jejuni
*One additional sample contained more than 9 log(10) copies of Camp. jejuni