In vitro Inhibition of Neuraminidase Activity of Influenza Virus (H5N2) by Methanolic Soluble Fraction of Ganoderma lucidum Extract
Published: April 25, 2014
By: Bala, U. Shamaki1*, El-Yuguda Abdul-Dahiru2, Fanna, I. Abdulrahman 3, Dr. A.O. Ogbe 4, Umar K. Sandabe 1
1 Department of Veterinary Physiology, Pharmacology and Biochemistry, Faculty of Veterinary Medicine, University of Maiduguri, Nigeria
2 Animal Virus Research Laboratory, Department of Veterinary Microbiology and Parasitology, Faculty of Veterinary Medicine, University of Maiduguri, Nigeria
3 Department of Chemistry, Faculty of Science, University of Maiduguri, Nigeria
4 Department of Animal Science, Faculty of Agriculture, Nassarawa State University, Lafia, Nigeria
International Journal of Modern Biology and Medicine, 2013, 4(3): 147-154. ISSN: 2165-0136. Received 4 August 2013, Received in revised form 26 September 2013, Accepted 1 October 2013, Published 16 October 2013.
2 Animal Virus Research Laboratory, Department of Veterinary Microbiology and Parasitology, Faculty of Veterinary Medicine, University of Maiduguri, Nigeria
3 Department of Chemistry, Faculty of Science, University of Maiduguri, Nigeria
4 Department of Animal Science, Faculty of Agriculture, Nassarawa State University, Lafia, Nigeria
International Journal of Modern Biology and Medicine, 2013, 4(3): 147-154. ISSN: 2165-0136. Received 4 August 2013, Received in revised form 26 September 2013, Accepted 1 October 2013, Published 16 October 2013.
Abstract
The anti-neuraminidase activity of different dilutions of methanolic soluble fraction of Ganoderma lucidum (GL) extract was tested against avian influenza virus (H5N2 strain) using chicken red blood cell (RBC). The endpoint titre of anti-neuraminidase activity of the methanolic fraction of GL was determined by observing the wells that do not elute after long incubation (≥ 1 hour) at room temperature or overnight at 4°C. Elusion inhibitory activities of the extract were observed at viral dilutions of up to 1:32 compared to control. The methanolic soluble fraction of GL extract has anti-neuraminidase activity by inhibition of elusion, and may be a potential agent against avian flu.
Keywords: Ganoderma lucidum; methanolic fraction; haemagglutination; elusion; neuraminidase; avian influenza virus; H5N2
1. Introduction
The classics (Chinese emperors) first used Zhi during the warring state period (475-221 BCE) and Lingzhi (Ganoderma lucidum or Reishi) during the Han dynasty (206BCE- 220CE) (Unschuld, 1985). Ganoderma lucidum comes in a variety of forms (Engelbrecht and Volks, 2005). Reishi occurs in six different colours but the red variety is the most commonly used and cultivated in many countries, and is generally regarded as the most potent and medicinal (Hsu et al., 1985). Some mushrooms are used for medicinal purposes or as antimicrobials. Examples are anti-smallpox mushroom, antibiotic mushroom, cytotoxic mushroom and libido enhancing mushroom (Wasser, 2005). Proteins from some mushrooms are known to enhance cell mediated immune response in vitro and in vivo (Guo et al., 2003). T-cell immune response characterized by secretion of interferon, cytokine stimulation and macrophage activity were enhanced by using mushrooms (Hattori et al., 2011). They also stimulate the growth of immune organs in Newcastle disease infected chickens (Wei et al., 1997).
The re-assortant properties of the virus (H5N2) with increasing host range (Hu et al., 2010), associated with reports of drug resistance development against current anti-influenza virus, and its being expensively unavailable and sometimes unaffordable, has stimulated research into alternative anti-influenza virus with neuraminidase as target (Pleschka et al., 2009; Hu et al., 2010; Costa et al., 2012) especially in African countries, where new diseases are easily imported. Medicinal properties of extracts from Ganoderma lucidum has been investigated against various disease conditions such as anti-tumor effect (Lin et al., 1991), anti-hypertensive activity (Jin et al., 1996), related to cholesterol lowering activity ((Kabir et al., 1988), anti-bacterial activity (Yoon et al., 1994), hepatoprotective activity (Wasser, 1997), anti-human immunodeficiency virus (HIV) activity (Gao et al., 2003), and anti-inflammatory potentials through regulating histamine-mediating allergies (Powell, 2006). However, there is dearth of information on the neuraminidase activity of Ganoderma lucidum extracts. This research is aimed at finding the anti-neuraminidase activity of methanolic soluble fraction from Ganoderma lucidum extract.
2. Materials and Methods
2.1. Preparation of the Methanolic Extract
Extracts was prepared by standard method as described by Trease and Evans (1997). Dried Ganoderma lucidum powder was weighed 1.5 kg, using a Metler balance (Toledo-PB 153, Switzerland) and placed in a thimble then into a Soxhlet chamber, to this was added 7.5 L of methanol and the mixture was steamed at 40 oC for 24 hours until the methanol fraction was completely extracted, the filtrate was evaporated within a period of 24 hours, using electric evaporator. A bitter tasting, chocolate coloured, sticky extract with a pH of 6.9 which dissolves in water to give a greenish-yellow colour was realized from the methanol fraction of the mushroom. It was weighed, and the yield was calculated to be 79.5 g (5.3%). The extract was weighed (0.2 g) and reconstituted with 10 mL of distilled water to prepare 200 mg/mL concentration, which was then used for the study.
2.2. Antigen Used for Neuraminidase Test
Influenza virus (H5N2 - OIE/FAO-Istituto zooprofilatico delle venezie Laboratory for AI Italy, Rome), was obtained from Animal Virus Research Laboratory at the Department of Veterinary Microbiology and Parasitology, Faculty of Veterinary Medicine, University of Maiduguri. This antigen was used as source of neuraminidase in the test against the methanolic soluble fraction of Ganoderma lucidum extract.
2.3. Haemagglutination Test for Influenza Virus (H5N2) and Anti-neuraminidase Assay
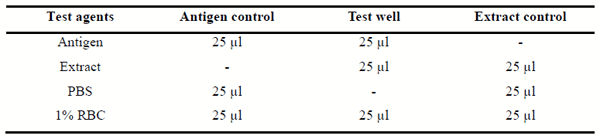
The neuraminidase (NA) activity of methanolic soluble fraction of Ganoderma lucidum extract was determined in vitro using modification of the methods described by Matrosovitch et al. (2004). Screening test was first conducted to see whether the antigen haemagglutinate and elutes the red cells and to see if the extract of Ganoderma lucidum can inhibit the elusion. The screening test was carried out using a microtitre plate, and 25 μL of antigen was added into the first and second wells and 25 μL of the extract was added into the second and third wells (Table 1). Another 25 μL of PBS (Phosphate Buffered Saline) was added to the first and third wells, finally, 25 μL of 1% chicken (RBCs) was added to all the three wells and the microtitre plate was incubated at room temperature for 30 min. The first well served as the antigen control, the second well served as the test well and the third well served as the extract control respectively. The plates were observed for haemagglutination. The plates were incubated further until the antigen well completely eluted. Positive anti-neuraminidase activity of the extract was indicated by non elusion of the test well (Well containing extracts, antigen and 1% RBC).
Extracts that were observed to possess anti-neuraminidase activity were titrated to end points titre against a constant dilution of the antigen. This titration was carried out by dispensing 25 μL PBS into all the wells in a row except the first well. The first well was dispensed with 50 μL of extract and 25 μL from that well was transferred into the second well and mix very well, and transferred another 25 μL from the second well into the next well and continue up to the second to the last well. Another 25 μL of appropriately diluted antigen (8 haemagglutinating units) was added to all the wells. Finally 25 μL of 1% chicken RBC was added to all the wells and the microtitre plate was incubated at room temperature for 30 min. The endpoint titre of anti-neuraminidase activity of the methanolic fraction was determined by observing the wells that do not elute after long incubation (≥ 1 hour) at room temperature or overnight at 4°C.
Table 1. Microtitre plate preparation for neuraminidase inhibition test
3. Results
The result obtained from methanolic extraction of Ganoderma lucidum gave a bitter tasting, chocolate coloured, gelatinous methanol extract which can dissolve in water to produce a greenish-yellow colour, which change to colourless solution at pH 5.78. The yield of the extract was calculated to be 79.5 g (w/w) which represents 5.3%. The in vitro haemagglutination inhibition (HI) and neuraminidase (NA) inhibitory activity of methanolic soluble solvent fraction of Ganoderma lucidum extract against influenza virus (H5N2) is presented in Table 2 and Plate 1. The result showed that in vitro neuraminidase inhibitory activity of the methanolic soluble fraction of the extract was observed at dilutions of up to 1:32 compared to control.
Table 2. Haemagglutination inhibition properties of methanolic soluble fraction of Ganoderma lucidum extract
Plate 1. Anti-neuraminidase activity of methanolic soluble fraction of Ganoderma lucidum extract on elution of influenza virus (H5N2) agglutinated chicken 1% RBCs. A = Antigen control, B = Extract control and C = Test well.
4. Discussion
The methanolic soluble fraction of Ganoderma lucidum extract under study showed anti-neuraminidase activity, with avian influenza virus (H5N2) inhibiting elusion, suggesting haemagglutination inhibition of 1% chicken red blood cells (RBCs) after 1 hour of incubation. This finding agrees with that of Matrosovitch et al. (2004), who reported inhibition of RBC haemagglutination after 1 hour of incubation, when the RBCs were subjected to different anti-influenza agents and other agents from Chinese Traditional Medicine. Neuraminidase inhibition by this fraction of the extract may also be due to activation of a low pH proton channel – M2 protein that mediates the interior of viral particles trapped in endosomes as reported by Rosenberg and Casarotto (2010). The activation of this proton channel may possibly be responsible for the different inhibitory pattern exhibited by the Ganoderma lucidum extracts. Report by Hu et al. (2010) suggested that this protein serves as gate that opens or closes, to allow entrance of macromolecules into cells, or disallow its entrance under low and high pH levels. The extract may have stopped the protons from entering the virions which then do not disintegrate. Lowering the pH can also destabilize the transmembrane helical packing and unlock a tryptophan gate, usually locked by intermolecular interactions thereby, admitting water to conduct protons, whereas the C-terminal base containing no extra cavity remains intact, and preventing disintegration of the proton thus inhibiting neuraminidase activity. Neuraminidase inhibitory activity of this mushroom can also be attributed to detectable quantities of flavonoids present. This compound and its derivatives are reported to form hydrogen bonds within the HA binding pockets, thus inducing non recognition and biding to host’s cells, therefore preventing spread of infection to cells as observed with H1N1 virions (Pinto and Lamb, 2006). Detectable quantities of flavonoids were found in both the methanol soluble fraction of the Ganoderma lucidum extract and may have accounted for the neuraminidase inhibitory activity observed in this fraction. Flavonoids derivatives and biflavonoids are also reported to exert NA inhibitory effect and suppress spread of influenza viruses (Liu et al., 2008, Costa et al., 2012).
5. Conclusions
The methanolic soluble fraction of Ganoderma lucidum extract inhibited the neuraminidase elusion of influenza virus (H5N2) agglutinated 1% chicken RBC suspension and could be a possible source of anti-influenza virus agent that can be used effectively to manage avian influenza infections in both man and animals.
Acknowledgements
We sincerely thank the University of Maiduguri management for sponsoring this research, and equally wish to appreciate the technical assistance of Mr. Andrew Ali of the Animal Virus Research Laboratory, Department of Microbiology and Parasitology, Faculty of Veterinary Medicine, University of Maiduguri.
References
Costa, S. S., Couceiro, J. N. S. S., Silva, I. C. V., Malyar, D. D. O. C., Coutinho, M. A. S., Camargo, L. M. M., Muzitano, M. F., and Vanderlinde, F. A. (2012). Flavonoids in the therapy and prophylaxis of flu: A patent review. Phytother., 22: 1111-1121.
Engelbrecht, K., and Volk, T. (2005). Ganoderma lucidum, Reishi or Ling Zhi, a fungus in oriental medicine. Tom Volk’s fungus of the month for arch 2005, pp. 1-4.
Gao, Y., Liu, W., Gao, H., Qi, X., Lin, H., Wang, X., and Shien, R. (2003). Effective inhibition of infectious bursal disease virus by DNA vector based RNA-interference. Antiviral Res., 79: 87-74.
Guo, F. C., Sanelkowski, H. F. J., Kwakkel, R. P., Williams, B. A., and Verstagen, M. W. A. (2003). Immunoactive medicinal properties of mushroom herb polysaccharide and their potential use in chicken diet. World Poultry Sci. J., 59: 127-440.
Hattori, M., el-Mekkawy, S., and Meselhy, M. R. (2011). Inhibitory effect of components from Ganoderma lucidum on the growth of human immunodeficiency virus (HIV), and the protease activity. Research Institute for Wakan-Yaku (Traditional Sino-Japanese Medicine), Toyana Medical and Pharmaceutical University, Japan.
Hsu, H. Y., Chen, Y. P., Shen, S. J., Hsu, C. S., Chen, C. C., and Chang, H. C. (1985). Oriental Materia Medica: A concise guide. Oriental Healing Arts Institute, Long Beach, CA, USA, pp. 640-641.
Hu, G., Wang, Y. F., Xu, J., Gu, Q., Liu, H. B., Xiao, P. G., Zhou, J., Liu, Y., Yang, Z., and Su, H. (2010). Anti-influenza agents from Traditional Chinese Medicine. Rev. Nat. Prod. Rep., 48: 1758-1780.
Jin, H., Zhang, G., Cao, X., Zhang, M., Long, J., Luo, B., Chen, H., Qian, S., Mori, M., and Wang, Z. (1996). Treatment of hypertension by lingzhi combined with hypotensor and its effects on arterial, arteriolar and capillary pressure microcirculation. In: Microcirculatory Approach to Asian Traditional Medicine: Strategy for the Scientific Evaluation. Excerpta Medica, International Congress, Series 1117; Elservier; Amsterdam, New York, pp. 131-138.
Kabir, Y., Kimura, S., and Tamura, T. (1988). Dietary effect of Ganoderma lucidum on blood pressure and lipids levels in spontaneously hypertensive rats (SHR) J. Nutr. Sci. Vitaminol., 34: 433-438.
Lin, C. N., Tome, W. P., and Won, S. J. (1991). Novel cytotoxic principles of formosan Ganoderma lucidum. J. Nat. Prod., 54: 998-1002.
Liu, A. L., Wang, H. D., Lee, S. M., Wang, Y. T., and Du, D. H. (2008). Structure-activity relationship of 25 flavonoids on their influenza virus neuraminidase inhibitory activity. Bioorg. Med. Chem., 16: 7141-7147.
Matrosovitch, M. N., Matrosovitch, T. Y., Gray, T., Roberts, N. A., and Klenk, H. D. (2004). Neuraminidase is important for the initiation of influenza virus infection in human airway epithelium. J. Virol., 78: 12665-12667.
Pinto, L. H., and Lamb, K. A. (2006). Activation of M2 protein inhibits neuraminidase activity in flu. J. Biol. Chem., 281: 8997-9000.
Pleschka, S., Stein, M., Schoop, R., and Hudson, J. B. (2009). Antiviral properties and mode of action of standardized Echinacea purpurea extract against highly pathogenic avian influenza virus (H5N1, H7N7) and swine origin H1N1 (S-OIV). Virol. J., 13(6): 196-197.
Powell, M. (2006). The use of Ganoderma lucidum in the management of histamine-mediated allergic response. Health Publications, 12: 1-2.
Rosemberg, M. R., and Casarotto, M. G. (2010). Measurement of affinity of amantadine and rimantidine to M2 ion cannels by use of surface resonance plasmon technique. Proc. Natl. Acad. Sci. USA, 107: 13866-13871.
Trease, G. E., and Evans, W. C. (1997). Textbook of Pharmacognosy, 14th Edn. W. B. Saunders Company Limited, London, UK.
Unschuld, P. U. (1985). Medicine in China: A History of Ideas. University of California Press, p. 112.
Wasser, S. P., and Weis, A. L. (1997). Medicinal Mushrooms. Ganoderma lucidum. (Curtis. Fr.) P. Karst. Nevo, E. (ed.), Peledfus Publication House, Haifa Israel, p. 39.
Wei, P., Li, Q. Z., and Hao, Y. Z. (1997). Effects of Astragalus monoghonas and Lentinus edodes polysaccharides on lipid peroxide (LPO) contents in mareks disease infected chicks. J. Trad. Chin. Med., 4: 3-4.
Yoon, S. Y., Eo, S. K., Kim, Y. S., Lee, C. K., and Han, S. S. (1994). Antimicrobial activity of Ganoderma lucidum extract alone and in combination with some antibiotics. Arch. Pharmacol. Res., 17: 138-442.
Related topics:
Authors:
AOgbe
Recommend
Comment
Share

Would you like to discuss another topic? Create a new post to engage with experts in the community.








.jpg&w=3840&q=75)