Prospect of Leaf Extracts on The Performance and Blood Profile of Monogastric – A Review
Medicinal plants are used to prevent, treat and promote healthy life in human and animals, their medicinal value are due to the presence of bioactive chemicals or secondary metabolites (phytochemicals) making it more suitable for animals with benefits of low cost and total safety. Phytochemicals are chemical compounds that occur naturally in plants and they perform a multiple biological activities such as antibacterial, antioxidants, anti-inflammatory, antioxidants, antifungal, hepato-protective, hypolipidemic and antiviral properties. The efficacy of phytochemicals in plants depends on their organic composition (tannin, flavonoids, terpenoids, saponin, phenols and alkaloids), method of extraction or processing, harvesting procedure, soil type, geographical location and level of maturity. Plants are also found to be loaded with minerals, vitamins and other nutrients needed for the growth and development of animals.
Keywords: Medicinal plants; leaf extract; phytochemicals; chemicals; nutrients.
Obikaonu, H.O., Okoli, M.A., Opara, I.C., Okoro, V.M.O., Ogbuewu, E.B., Etuk, E.B and Udebidie, A.B.I. (2011). Haematological and serum biochemical indices of starter broilers fed neem leaf meal. Online Journal of Animal and Feed Research, 4: 150-154.
Tekeli, A., Çelik, L.,Kutlu, H.R and Görgülü, M. (2014). Effect of dietary supplemental plant extracts on performance, carcass characteristics, digestive system development, intestinal microflora and some blood parameters of broiler chicks. British Poultry Science. 57 (2):99-106.
Alabi, O.J., Malik, A.D., Ng’ambi, J.W., Obaje, P and Ojo, B.K. (2017). Effect of aqueous Moringa olifera leaf extracts on growth performance and carcass characteristics of broiler chicken. Brazilian Journal of Poultry Science, 19(2):273-280.
Rates, S.M.K. (2001). Plants as source of drugs. Journal of Toxicology, 29: 603-613.
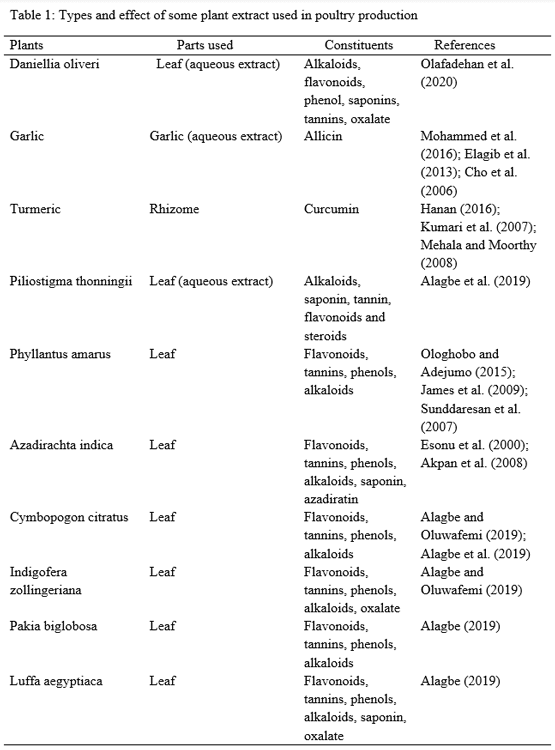
Alagbe, J.O., Sharma, D.O and Xing, L. (2019). Effect of aqueous Piliostigma thinningii leaf extracts on the performance and serum biochemical indices of broiler chicken. Noble International Journal of Agriculture and Food Technology, 1(2): 62-69.
Liu Y, Song M, Che TM, Bravo D, Pettigrew JE (2012). Anti-inflammatory effects of several plant extracts on porcine alveolar macrophages in vitro Journal of Animal Science, 90:2774–2783
Kassa Shawle. , Mengistu Urge and Getachew Animut (2016). Effect of different levels of Lepidium sativum L. on growth performance, carcass characteristics, hematology and serum biochemical parameters of broilers. SpringerPlus (2016) 5:1441
Lillehoj, H.S and Lee, K.W (2012). Immune modulation of innate immunity as alternatives-to-antibiotics strategies to mitigate the use of drugs in poultry production. Journal of Poultry Science 91:1286–1291.
Faluyi, O.B and Agbede, J.O.( 2018). Immuno-modulatory activity of Aqueous leaf extract of Moringa oleifera in broiler chickens. International Journal of Environment, Agriculture and Biotechnology, 3(1): 49-54.
Haruna, S.S., Ahmed, O and Abdullahi, S.K. (2016). Phytochemical, proximate and mineral analysis of Solanum incanum fruit. International Journal of Chemical, Mineral and Environmental Research, 3(1):8-13.
Tsado, N.A., Lawal, B., Santali, E.S., Mohammed, A.S., Balarabe, M.M., Ibrahim, H.A and George, J.J. (2015). Phytochemical and acute toxicity profile of aqueous and methanolic extracts of Crateva adansonii leaves in Swiss Albino Rats. Asian Journal of Biochemistry, 10(4): 173-179.
Asl, M.N and Hosseinzadeh, H. (2008). Review of pharmacological effects of Glycyrrhiza spp and its bioactive compounds. Phytother. Res., 22:709-724.
Asquith, T.N and Butler, L.G. (1986). Interactions of condensed tannins with selected proteins. Journal of Phytochemistry, 25:1591-1593.
Igboko, D.O. (1983). Phytochemical studies on Garcinia kola Heckel. M.Sc Thesis, University of Nigeria Nsukk
Lawal, M., Wasagu, R.S.U and Ladan, M.J. (2005). Hepatoxicity risk assessment of neem seed extract using Albino rats. Biol. Environ. Sci. J. Tropics, 2:36-38.
Ojewuyi, O.B, Ajiboye, T. O, Adebanjo, E. O, Balogun, A, Mohammed, A.O (2014). Proximate composition, phytochemical and mineral contents of young and mature Polyalthia longifolia Sonn.leaves. Fountain Journal of Natural and Applied Sciences, 3(1): 10 – 19.
Adisa, R.A., Oke, J.M., Olomu, S.A., Olorunsogo, O.O. (2004). Inhibition of human haemoglobin glycosylation by flavonoidcontaining leaf extract of Cnestis ferruginea. J. Cameroon Acad. Sci. 4:351- 359.
Cheeke, P. R. (2000). Actual and potential applications of Yucca schidigera and Quillaja saponaria saponins in human and animal nutrition. J Anim Sci. 77:1-10.
Galeotti, F., E. Barile, P. Curir, M. Dolci and V. Lanzotti, (2008). Flavonoids from carnation (Dianthus caryophyllus) and their antifungal activity. Phytochem. Lett. 1: 44.
Enzo, A.P. (2007). Traditional plants and herbal remedies used in the treatment of diarrheal disease: Mode of action, quality, efficacy and safety considerations. In: Ahmad I, Aqil F, Owais M, editors. Modern Phytomedicine Turning Medicinal Plants in to Drugs. WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim, 248-260.
Saleem, R., Ahmed, M., Ahmed, S. I., Azeem, M., Khan, R. A., Rasool, N. et al. (2005). Hypotensive activity and toxicology of constituents from root bark of Polyalthia longifolia var.pendula. Phytother Res. 19:881–4
Babajide, S. O., Oluwalana, S. A., Ajala, M. O. and Folarin, M. O. (1999). Phytochemical Screening of seeds of Acacia nilotida (Schum and Thonn). The Boprospector. 1(2):27-31.
Alagbe, J.O and Omokore, E.A. (2019). Effect of replacing soya bean meal with Indigoferazollingeriana leaf meal on the performance and carcass characteristics of growing rabbits. International Journal of Multidisciplinary Research and Development. 6(5): 74-77.
Sudipta Talukder, Md. Mehedi Hasan, Zakaria Al Noman, Yousuf Ali Sarker, Torun Kumar Paul and Mahmudul Hasan Sikder (2018). Influence of dietary supplementation of ginger powder at different levels on growth performance, haematological profiles, slaughter traits and gut morphometry of broiler chickens. South African Journal of Animal Science, 48 (6): 999-1008.
Faizi, S., Mughal, N. R., Khan, R. A., Khan, S. A., Ahmad, A., Bibi N. et al. (2003b). Evaluation of the antimicrobial property of Polyalthia longifolia var.pendula: isolation of a lactone as the active antibacterial agent from the ethanol extract of the stem. Phytother Res. 17:1177–81.
Vera Perricone., Marcello Comi., Carlotta Giromini., Raffaella Rebucci., Alessandro Agazzi ., Giovanni Savoini and Valentino Bontempo. Green tea and Pomegranate extract administered during critical moments of the production cycle improves blood antiradical activity and alters cecal microbial ecology of broiler chickens. Animals 20 (10): 780-785.
Ngozi, K.A., Chimaraoke, O., Chima, A.E.E and Jennifer, C.O. (2017). Phytochemical, proximate analysis of aqueous extract of Ficus capensis leaves in South Eastern Nigeria. Journal of Applied Pharmaceutical Science, 7(3):117-122.
Alagbe, J.O (2019). Haematology, serum biochemistry, relative organ weight and bacteria count of bacteria given different levels of Luffa aegyptiaca leaf extracts. International Journal of Advanced Biological and Biomedical Research, 7(4):382-392.
Alagbe, J.O and Oluwafemi, R.A. (2019). Performance and haematological parameters of broiler chicks gives different levels of dried lemon grass (Cymbopogoncitratus) and garlic (Allium sativum) extract. Research in: Agricultural and Veterinary Sciences. 3(2): 102 – 111.
Alagbe, J.O., Olanrewaju, A., Adewemimo, A and Tanimomo, B.K. (2019). Carcass, caecal microbial population and immune parameters of broilers given different levels of mixed lemon grass ((Cymbopogoncitratus) and garlic (Allium sativum) extract.Academic Journal of Life Sciences. 5(11): 107-111
Dudareva, N., Pichersky, E and Gershenzon, J (2004). Biochemistry of plant volaties. Journal of Plant Physiology, 135:1893-1902.
Igidi, O.J and Edene, C.E. (2014). Proximate and phytochemical compositions of Napoleona vogelii hook fruit. International Journal of Engineering and Science, 3(6):45-51.
Saxena, M., Saxena, J., Nema, R., Singh, D and Gupta, A (2013). Phytochemistry of medicinal plants. Journal of Pharmacognosy and Phytochemistry Centre of Microbiology and Biotechnology Research and Training, Bhopal, India, 8192(1): 168-182.
Alagbe, J.O. (2020). Performance, hematology and serum biochemical parameters of weaner rabbits fed different levels of fermented Lagenaria brevifora whole fruit extract. Advances in Research and Reviews, 2020, 1:5.
Sczkowski, C.P., Kalinowska, M and Wojciechowski, Z. (1998). The 3-O-glucosylation of steroidal saponin and alkaloids in egg plants; evidence of two separate glycosyl transferences. Journal of Phytochemistry, 48:1151-1159.
Hilal Ürüsan and Canan Bölükbasi (2017). Effects of dietary supplementation levels of turmeric powder (curcuma longa) on performance, carcass characteristics and gut micro flora in broiler chickens. Journal of Animal & Plant Sciences, 27(3): 732-736.
Alagbe, J.O. (2019). Effects of dried Centellaasiatica leaf meal as a herbal feed additive on the growth performance, haematology and serum biochemistry of broiler chicken. International Journal of Animal Research. 3(23): 1-12.
Ologhobo, A.D and Adejumo, I.O. (2015). Haematological response and serum biochemical profile of broiler finishers fed Oxytetracycline and Stonebreaker leaf meal. British Biotechnology Journal, 7(1):51-56.
James, D.B., Owolabi,O.A., Elebo, N and Odemene, L. (2009).Glucose tolerance test and some biochemical effect of Phyllantus amarus aqueous extract on normalglycemic albino rats. African Journal of Biotechnology, 8(8): 1637-1642.
Hanan, E.A. (2014). Effect of different levels of turmeric supplementation on broiler performance, carcass characteristics and bacteria count. Egyptian Poultry Science Journal, 35(1):25-39.
Kumari, P.,Gupta, M.K., Ranjan, K.K., Singh R. (2007). Curcuma longa as feed additive in broiler birds and its patho physiological effects. Indian Journal of Experimental Biology, 45:272-277.
Mahela, C and Moorthy, M. (2008). Production performance of broilers fed Aloe vera with turmeric. International Journal of Poultry Science, International Journal of Poultry Science, 7:852-856.
Mohammed, I.E., Mosad, A.S., Mohammed, M.S and Adel, H. (2016). Growth performance, immune response, blood serum parameters, nutrient digestibility and carcass traits in broiler chickens as affected by dietary supplementation of garlic extract. Alexandria Journal of Veterinary Sciences, 49(2): 50-64.
Cho, S.J., Rhee, D.K and Pyo, S. (2006). Allicin a major components of garlic, inhibits apoptosis of macrophage in depleted nutritional state. Journal of Nutrition, 22(12):77-78.
Elagid, H.A.A., El-Amin, W.I.A., Elamin, K.M and Malik, H.E.E. (2013). Effect of dietary garlic supplementation as feed additive on performance and blood profile of broiler chicken. Journal of Animal Science, 3(2):58-64.










.jpg&w=3840&q=75)