Occurrence of Avian Influenza H5N1 among Chicken, Duck Farms and Human in Egypt
Avian influenza (AI) H5N1 virus consider a potential threat to the poultry industry with sever zoonotic effect associated with a high risk on human being associated with the poultry production. The disease become endemic in Egypt and cause more than 359 confirmed human infection cases in the last few years. Samples (serum and tissues) collected from 160 poultry farms that suspected to be infected with H5N1. Samples were collected from 75 broiler chicken farms, 55 broiler duck farms and 30 layer duck farms. As well as 115 human samples (serum and throat swabs) collected from persons suffering from respiratory manifestations and have a history of contact with infected birds. Samples were examined using Haemagglutination inhibition (HI) test and Reverse Transcription Real Time PCR (RT- qPCR) for detection of avian influenza H5N1 virus. HI test detect H5N1 antibodies in 71 poultry samples out of 160 (44.4%). While, RTqPCR detect H5N1 in 13 out of 160 samples (8.1%). On the other hand, HI test detect H5N1 antibodies in six human sample out of 115 human cases (5.2%) while, RT- qPCR detect H5N1 in two human samples out of 115 human cases (1.7%). There was an increase in rate of highly pathogenic avian influenza transmission from poultry-to-human. Adaption of AI virus was increased to duck flocks with higher percentage of vaccinal escape. Thus, there is a need to give more attention to the vaccination programs and increasing the usage of the vaccines prepared from Egyptian H5N1 virus or update the current vaccines with the isolates representing the circulating virus in the Egyptian market. All these measures will increase the protection level in poultry farms and intern decrease the risk of human infection rates.
Keywords: H5N1, Escape Mutant, Vaccinal Failure, Zoonotic AIV
- The current situation of poultry farm infection by H5 virus in West Egypt; (Alexandria, El-Garbia and El-Behera governorates) and the level of protection in vaccinated and non-vaccinated farms.
- The level of infection associated with heterologous vaccines.
- 3 - Human infection cases
- RNA extraction was performed on the supernatant of tissues homogenate, nasal swabs that were taken from the dead bird and throat samples using a QIAamp Viral RNA Mini Kit (Qiagen GmbH, Hilden, Germany) according to the manufacturer’s procedure
- One-step RT-PCR: PCR products Influenza type-A virus was screened using RT-qPCR assay that targeted the influenza Matrix gene. Samples were amplified using One-step (Reverse Transcription and Amplification) Real Time RT-PCR Kit (Qiagen, Germany) for detection of type-A avian influenza viruses using; Exicycler thermal block Real-Time PCR device (Bioneer, Korea), targeting the matrix gene through primers and probe mentioned by Spackman et al. (2002); Forward primer: 5′-AGA TGA GTC TTC TAA CCG AGG TCG-3′, Reverse primer: 5′-TGC AAA AAC ATC TTC AAG TCT CTG-3′and probe: 5′ FAM-TCA GGC CCC CTC AAA GCC GA-TAMRA-3


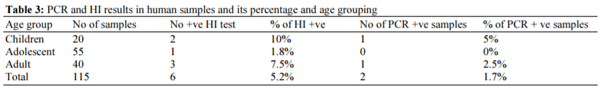
Abdelwhab, E. and H. Hafez, 2011. An overview of the epidemic of highly pathogenic H5N1 avian influenza virus in Egypt: Epidemiology and control challenges. Epidemiology Infection, 139: 647-657.
Alexander, D.J., 2000. A review of avian influenza in different bird species. Veterinary Microbiology, 74: 3-13.
Alexander, D.J., 2007. An overview of the epidemiology of avian influenza. Vaccine, 25: 5637-44.
Alexander, D.J., R.J. Manvell, R. Irvine, B.Z. Londt and B. Cox et al., 2010. Overview of incursions of Asian H5N1 subtype highly pathogenic avian influenza virus into Great Britain, 2005-2008. Avian Diseases, 54: 194-200.
Ameji, O., L. Saidu and P. Abdu, 2016. Sero-prevalence of avian influenza in poultry in Kogi State, Nigeria. Science, 6: 1-6.
Arafa, A., D. Suarez, S.G. Kholosy, M.K. Hassan and S. Nasef et al., 2012. Evolution of highly pathogenic avian influenza H5N1 viruses in Egypt indicating progressive adaptation. Arch Virol., 157: 1931-47. Chen, H., 2009: Avian influenza vaccination: The experience in China. Rev. Sci. Tech., 28: 267-74.
Eid, H.I., A.M. Algammal, S.A. Nasef, W.K. Elfeil and G.H. Mansour, 2016. Genetic variation among avian pathogenic E. coli strains isolated from broiler chickens. Asian J. Animal Vet. Advances, 11: 350-356.
Fasanmi, O.G., I.A. Odetokun, F.A. Balogun and F.O. Fasina, 2017. Public health concerns of highly pathogenic avian influenza H5N1 endemicity in Africa. Veterinary World, 10: 1194.
Fasina, F., V. Ifende and A. Ajibade, 2010. Avian influenza A (H5N1) in humans: Lessons from Egypt. Euro Surveill 15: 19473.
Gilbert, M., P. Chaitaweesup, T. Parakamawongsa, S. Premashthira and T. Tiensin et al., 2006. Freegrazing ducks and highly pathogenic avian influenza, Thailand. . Emerging Infectious Dis., 12: 227-34.
Grund, C., E.S.M. Abdelwhab, A.S. Arafa, M. Ziller and M.K. Hassan et al., 2011. Highly pathogenic avian influenza virus H5N1 from Egypt escapes vaccineinduced immunity but confers clinical protection against a heterologous clade 2.2.1 Egyptian isolate. Vaccine, 29: 5567-5573.
Henning, J., H. Wibawa, J. Morton, T.B. Usman and A. Junaidi et al., 2010. Scavenging ducks and transmission of highly pathogenic avian influenza, Java, Indonesia. Emerg. Infect. Dis., 16: 1244-50.
Huo, X., R. Zu, X. Qi, Y. Qin and L. Li et al., 2012. Seroprevalence of avian influenza A (H5N1) virus among poultry workers in Jiangsu Province, China: An observational study. BMC Infect. Diseases, 12: 1.
Kandeel, A., S. Manoncourt, E. Abd el Kareem, A. Mohamed Ahmed and S. El-Refaie et al., 2010. Zoonotic transmission of avian influenza virus (H5N1), Egypt, 2006–2009. Emerg. Infect. Dis., 16: 1101-1107.
Kaoud, H., H. Hussein, A. El-Dahshan, H. Kaliefa and M. Rohaim, 2014. Co-circulation of avian influenza viruses in commercial farms, backyards and live market birds in Egypt. Int. J. Vet. Science Med., 2: 114-121.
Kayali, G., A. Kandeil, R. El-Shesheny, A.S. Kayed and A.M. Maatouq et al., 2016. Avian Influenza A(H5N1) Virus in Egypt. Emerging Infect. Dis., 22: 379-388.
Kayali, G., S.F. Setterquist, A.W. Capuano, K.P. Myers and J.S. Gill et al., 2008. Testing human sera for antibodies against avian influenza viruses: Horse RBC hemagglutination inhibition vs. microneutralization assays. J. Clinical Virology, 43: 73-78.
Kayed, A., A. Kandeil, R. El Shesheny, M. Ali and G. Kayali, 2016. Active surveillance of avian influenza viruses in Egyptian Poultry 2015. Eastern Mediterranean Health J., 22: 557.
Lee, C.W. and D.L. Suarez, 2005. Avian influenza virus: Prospects for prevention and control by vaccination. Anim. Health Res. Rev., 6: 1-15.
Madsen, J.M., N.G. Zimmermann, J. Timmons and N.L. Tablante, 2013. Avian influenza seroprevalence and biosecurity risk factors in Maryland backyard poultry: A cross-sectional study. PLoS One, 8: e56851.
OIE, 2015. Manual of diagnostic tests and vaccines for terrestrial animals. OIE, Rome, Italy.
Peyre, M., H. Samaha, Y.J. Makonnen, A. Saad and A. Abd-Elnabi et al., 2009. Avian influenza vaccination in Egypt: Limitations of the current strategy. J. Molecular Genetic Med., 3: 198-204.
Spackman, E., D.A. Senne, T. Myers, L.L. Bulaga and L.P. Garber et al., 2002. Development of a real-time reverse transcriptase PCR assay for type a influenza virus and the avian H5 and H7 hemagglutinin subtypes. J. Clinical Microbiol., 40: 3256-3260.
Subbarao, K., A. Klimov, J. Katz, H. Regnery and W. Lim et al., 1998. Characterization of an avian influenza A (H5N1) virus isolated from a child with a fatal respiratory illness. Science, 279: 393-6.
Swayne, D.E. and J.R. Glisson, 2013. Diseases of poultry. Wiley-Blackwell, Ames, Iowa.
Swayne, D.E., 2017. Animal influenza. John Wiley and Sons, Inc, Ames, Iowa.
Swayne, D.E., G. Pavade, K. Hamilton, B. Vallat and K. Miyagishima, 2011. Assessment of national strategies for control of high-pathogenicity avian influenza and low-pathogenicity notifiable avian influenza in poultry, with emphasis on vaccines and vaccination. Rev. Sci. Tech., 30: 839-70.
Tong, S., Y. Li, P. Rivailler, C. Conrardy and D.A.A. Castillo et al., 2012. A distinct lineage of influenza A virus from bats. Proceedings of the National Academy Sci., 109: 4269-4274.
van der Goot, J.A., M. van Boven, S.A. van de Water, G.P. Sandra and M.C. de Jong et al., 2008. Transmission of highly pathogenic avian influenza H5N1 virus in Pekin ducks is significantly reduced by a genetically distant H5N2 vaccine. Virology, 382: 91-7.
Wang, H., Z. Feng, Y. Shu, H. Yu and L. Zhou et al., 2008. Probable limited person-to-person transmission of highly pathogenic avian influenza A (H5N1) virus in China. Lancet, 371: 1427-1434.
WHO, 2002. WHO Manual on Animal Influenza Diagnosis and Surveillance, WHO/CDS/CSR/NCS/2002.5 Rev. 1.
WHO, 2006. Confirmed human cases of avian influenza A (H5N1). Geneva, Switzerland: World Health Organization. World Health Organization, Geneva, Switzerland.
WHO, 2015. Cumulative number of confirmed human cases for avian influenza A(H5N1) reported to WHO, 2003–2015 [cited 2015 Apr 15]. In W. H. Organization (Ed.): EN_GIP_20150303. WHO, World Health Organization.
WHO, 2018. Cumulative number of comfirmed human cases for avian influenza A (H5N1) reported to WHO 2003-2018.
Wibawa, H., J. Bingham, H. Nuradji, S. Lowther and J. Payne et al., 2014. Experimentally infected domestic ducks show efficient transmission of Indonesian H5N1 highly pathogenic Avian Influenza virus, but lack persistent viral shedding. Plos One.
Wlliams, S.M., 2016. A laboratory manual for the isolation, identification and characterization of avian pathogens. American Association of Avian Pathologists, Jacksonville, Fl.
Zheng, T., B. Adlam, T. Rawdon, W. Stanislawek and S. Cork et al., 2010. A cross-sectional survey of influenza A infection and management practices in small rural backyard poultry flocks in two regions of New Zealand. New Zealand Vet. J., 58: 74-80.









.jpg&w=3840&q=75)