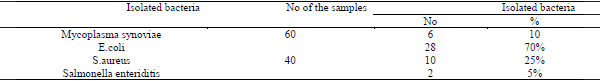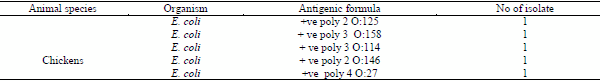Introduction
Bacterial arthritis in poultry commonly occurred after septicemia or localized infection to the joints is reported to be associated with many bacterial agents including Erysipelothrix, Listeria, Mycoplasma, Staphylococcus, and Escherichia (Mohan et al., 2002).
Arthritis is most commonly caused by Staphylococcus aureus (McNamee and Smyth, 2000), and sometimes involved Escherichia coli (Chansiripornchai, 2009), which is of veterinary importance in broiler breeders.
Mycoplasma synoviae causes a respiratory disorder and infectious synovitis in chicken especially when Mycoplasma synoviae combines with other respiratory virus infection, causing significant drop in egg production beside contamination of carcasses due to accumulation of the viscous creamy to grey exudates involving synovial membranes of the tendon sheath, joint, keel bursa and extend even to muscles and air sacs (Kleven, 1997; Ley et al., 2003).
Isolation and identification of Mycoplasma synoviae isolates are of critical importance. First isolation of Ms was from synovial sheaths and joints from commercial chicken (Morrow et al., 1990) also it was isolated from tracheal swabs as (Bradbury et al., 2001; Poveda et al., 1990; Wissman and Parsons, 1996).
For the first time in Iran, the primers complementary to the single-copy conserved 5' end of vlhAgene were used for detection of MS by PCR. Results obtained from serology, isolation, and PCR using primers related to 16s rRNA and vlhA genes were analyzed and compared. PCR results, in addition to identification of Mycoplasma sp, revealed variable sizes of 350-400 bp among standard strain, vaccine strains, and Iranian field isolates. The findings of this study demonstrated that the vlhA gene-targeted PCR is a sensitive and specific test for detection of M. synoviae, and an efficient tool for primary typing of its different strains (Ghafouri et al., 2011).
E.coli localization in bones and synovial tissue is a common sequel of colisepticemia affecting birds likely have insufficient resistance to completely clear bacteria (Huff et al., 2000). E.coli could be colonized in the vesicular sprouts that invade the physis of growing bone provoking an inflammatory condition, that resulting in osteomylities (Saif et al., 2011).The ibeA gene plays role in the pathogenesis of colibacillosis through invasion assays. After the deletion of the ibeA gene, these authors noted a decrease in virulence(Germon et al., 2005).Increase serum survival gene is the most important and widely distributed virulence marker of APEC (Ammar et al., 2011).
Staphylococcus aureus is the most common cause of bacterial arthritis in broiler breeder chickens. They recorded 51.1% mortality in broilers with lesion of swollen joints, gaseous exudates, cartilage injury, and synovial membrane thickening with infiltration of inflammatory cells(Gu et al., 2013).
The importance of the fibrinogen-bindin adhesin clumping factor A (clfA) in the pathogenesis of Staphylococcus aureus septic arthritis was examined in an animal model (Josefsson et al., 2001). The role of the collagen-binding function of CNA was examined in a mouse model of septic arthritis by comparing the virulence of isogenic strains of S. aureus expressing (1) wild-type CNA, (2) a truncated form of CNA (CNA35) with a higher affinity for collagen than the wild type, (3) CNA35 containing a single point mutation resulting in loss of collagen binding, (4) CNA lacking the collagen-binding domain, and (5) the collagen-binding domain of ACE (adhesion of collagen from Enterococcus faecalis). Results and conclusions: the results provide, for the first time, direct evidence that the virulence of CAN depends on its collagen-binding ability. Collagen binding facilitated early colonization of the joints of mice(Xu et al., 2004).
The aim of this study was planned to identify Mycoplasma synovia and other associated bacteria causing arthritis in chickens as well as detection of some virulence genes of isolated bacteria.
2. Materials and methods
2.1. Sampling:
A total of 100 samples (60 samples for Mycoplasma synoviae isolation and 40 samples for isolation other bacteria) from chicken with arthritis symptoms.
2.2. Isolation and identification of Mycoplasma synoviae:
A total of 60 collected swabs were inoculated into sealed sterilized tubes containing 2-3 ml of Frey's broth medium then incubated at 37Cfor 3-5 days and examined daily for acidity indicated by change of the color of medium from red to yellowish-orange (Kleven, 1997). The positive inoculated tubes were cultured on Frey's agar medium for 2-3 win sealed container supplied with damp cotton for increasing Humidity and lighted candle for C02 production and O2 exhaustion in the container atmosphere, the incubated plates were examined daily for the growth of colonies by dissecting microscope to demonstrate the characteristic colony.
2.3. Isolation and identification of other bacteria:
A total of 40 Bacteriological swabs and synovial fluid were inoculated into tryptic soy broth and incubated at 37C for 18hr then subcultured into 5% blood agar base, nutrient agar, Eosine Methylene agar, X.L.D, Mannitol salt agar, MacConkey agar and incubated at 37 C for 24-48hr .suspected colony from different media were picked up and subjected for morphological and biochemical identification (Quinn et al., 2011).
Serological identification of E.coli: The serological identification was done at the department of serological unit, Anima health research institute. Cairo. Using available polyvalent and monovalent E. coli antisera.
2.4. Molecular identification of isolated bacteria causing arthritis:
1-DNA extraction:
a.Mycolasma synoviae: DNA was extracted from Mycoplasma Broth using QIAamp DNA Mini Kit.according to the manufacturer instructions.
b.Staphylococcus .aureus and E.coli: Extraction of DNA by boiling method (Sambrook and Russell, 2001)
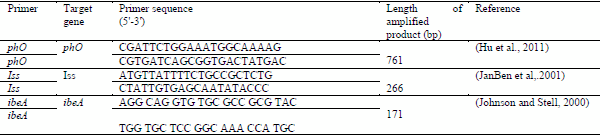
2- The conventional PCR primers were used in this study showed in this table (1, 2, 3)
Table (1): Oligonucleotide primers encoding for Mycoplasma synoviae :
Table (2): Oligonucleotide primers encoding for Staphylococcus aureus
Table (3): Oligonucleotide primers encoding for E.coli:
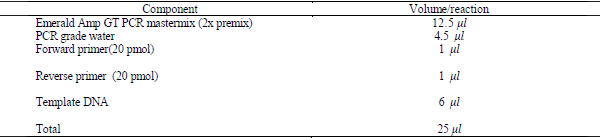
3-Preparation of PCR Master Mix for
a. Simple PCR: according to Emerald Amp GT PCR mastermix (Takara) Code No. RR310Akit as shown in table (4):
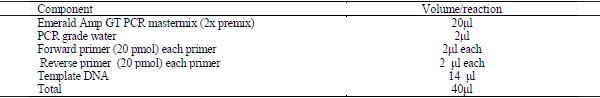
b. b-Multiplex PCR protocol for ibeA and iss genes:
c. According to Emerald Amp GT PCR mastermix (Takara) Code No. RR310Akit as shown in table (5).
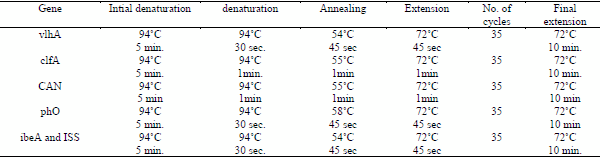
d. 4-Cycling conditions of the primers during cPCR :
e. Temperature and time conditions of the primers during PCR are shown in Table (6)
Table (4): component of PCR reaction of simple PCR
Table(5):component of multiplex PCR reaction for gene (ibeA,iss)
Table (6):Different PCR condition of different primers
5-DNA Molecular weight marker:
The ladder was mixed gently by pipetting up and down. Six μl of the required ladder were directly loaded.
6-Agarose gel electrophoreses (Sambrook and Russell, 2001)
3. Result and discussion
In Egypt, the swollen arthritic joint in broiler is a matter of economic losses as leads to inability of the chicks to obtain food and water resulting in loss of weight and even mortalities. The annual losses due to skeletal problems in united states were 80$ to 120$ million dollar (Sullivan, 1994) .so the aim of this study to identify bacteria causing arthritis.
In this study, Mycoplasma synoviae was isolated from arthritic samples at a percentage of 10%, although Mycoplasma synoviae could not be isolated by (Bkheet, 2011), who attributed this result to the septic arthritis and pyogenic organisms that present in the joint which may inhibit the Mycoplasma localization within joints and therapeutic may lead to these result, on the other hand, some others could detect 17.8% incidence of Mycoplasma as a causative agent of arthritis in chicken (Abd El-naser et al., 1994). Lower incidence was recorded 4.04% for MG and .8% for Ms (Shaker, 1995). The higher incidence of Mycoplasma synoviae in this study was due to the high susceptibility of chicken at young age.
The results of identification of other bacteria isolated from examined arthritic samples cleared that , the most commonly isolated bacteria were E. coli, Staphylococus aureus, Salmonella enterica which were isolated at a percentage of 26%, 13%, and 3.3%, respectively .The obtained results were higher than that described by (Mamza et al., 2010), who isolate E.coli and Staphylococcus aureus from hock joint and digital pad samples of chicken were isolated at a percentage of 4.2% ,8.1% from hock ioint and 14.3%,10.7% from digital pad respectively. These results were lower than that reported by (Rasheed, 2011), who isolate Staphylococcus aureus, E.coli, at a percentage of 50.98% and 7.8% respectively .This disagreement may be due to joint samples did not have any injuries or wounds , tightly close and opened under sterile condition so that the percentage of E.coli more than S.aureus and may be due to breed , age and environment.
Table (7): Results of typing bacteria associated with Mycoplasma synoviae isolated from arthritic samples:
Table (8): Results of serotyping of E. coli isolate :
in this study, serotyping of six E.coli isolated from arthritic samples of chicken indicated that the E.coli isolates were O114, O146, O128, O111, O27, O158 and O125. The serotype O125 and O146 nearly similar to the serotype obtained by (Abd El Tawab et al., 2014).
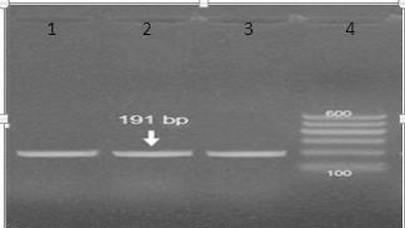
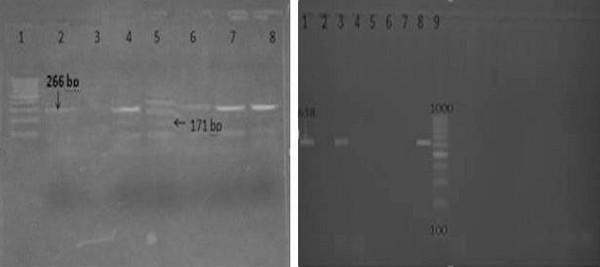
Results of amplification of Mycoplasma synoviae (vlhA) coding gene by using PCR: six isolates of Postive Ms broth culture were tested for vlhA using PCR technique. The specificity of the primers was confirmed by positive amplification of fragment with the extracted DNA as shown in figure(1). Out of 6 tested isolates, Three isolates (50%) were positive for the vlhA (figure1).
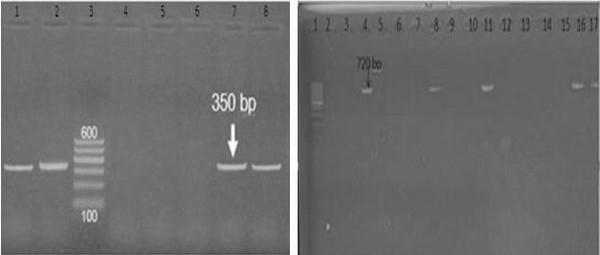
Results of amplification of E.coli (phoA) coding gene by using PCR: Sixteen isolates of biochemically identified E.coli were randomly studied for detection of phoA gene using PCR technique. The specificity of the primers was confirmed by positive amplification of fragment with the extracted DNA of the bacterial isolate as shown in (figure 2). Out of 16 tested isolates, Six isolates (37.5%) were positive for the phoA gene (figure 2). The PCR assay yielded amplified products of 720 bp specific for (phoA) gene;
Results of amplification of E.coli virulence genes by multiplex PCR (iss, ibeA): Iss and ibeA genes were detected in 6 pathogenic E. coli photo(3): Six isolated E.coli used for detection of virulence genes (iss, ibeA) which revealed that All 6(100%) isolate were positive for iss gene while 2 (33,3%) isolate were positive for ibeA.
The obtained results showed that presence of iss gene in E.coli isolated from arthritis with a percentage of 100% agree with the result mentioned by (Abd El Tawab et al., 2014), who recorded that PCR for amplification of iss gene of E. coli was 6(100%) of 6 APEC isolates and higher than the result that obtained by (Jeong et al., 2012) who mentioned that PCR for amplification of iss gene of E. coli was (78.2%) of APEC isolates. This study detected presence of IbeA gene in E.coli isolated from arthritis with a percentage of 33.3%.this result is similar to the result that obtained by (Cunha et al., 2014), who said that percent of PCR for amplification of IbeA gene of E. coli was (31%) of APEC isolates. and higher than the result obtained by (Wang et al., 2010) who identified ibeA gene with a percentage (10.6%) by using PCR.
Results of amplification of S. aureus clumping factor A (clfA) coding gene by using PCR which Eight isolates of biochemically identified S. aureus were randomly studied for detection of clumping factor gene using PCR technique. Out of 8 tested isolates, three (37.5.%) were positive The specificity of the primers was confirmed by positive amplification of fragment with the extracted DNA of the bacterial isolate as shown in figure (4)
In this study, the percentage of clfA gene was 37.5% which is lower than the result obtained by (Contreras et al., 2012) who found that clfA gene with percentage of 100%. Results of amplification of S. aureus collagen adhesin (cna) coding gene by using PCR: Collagen adhesin (cna) genes detected in 6 pathogenic S.aureus isolated from Arthiritic samples (figure5).
In this study, the percentage of cna gene was 100%, this result confirms (Xu et al., 2004) who said that cna is a virulance factor in septic arthritis. this result is higher than obtained from (Arciola et al., 2005), who found that cna gene with percentage of 46% and (Contreras et al., 2012) who found that cna gene with percentage of 78.1%.
Figure (5): Agarose gel electrophoresis patterns showing PCR amplification products for the S. aureus collagen adhesion (cna) coding gene. Lane 4, DNA molecular size marker (100 bp ladder), lane 1,2,3 was positive for coding gene showing amplification, lane 5 control positive. The PCR assay yielded amplified products of 191 bp specific for (cna) gene.
This article was originally published in Alexandria Journal of Veterinary Sciences 2016, Apr. 49 (2): 163-169 ISSN 1110-2047. DOI: 10.5455/ajvs.205876. This is an Open Access article licensed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-sa/3.0/).
4. Reference
Abd El-naser, A.A., Mahmoud, M., El-shabeeny, L.M., Abbas, A.A., 1994. Studies on majors bacterial agents causing arthiritis in chicken. Vet Med.J. Giza 42: 227- 285.
Abd El Tawab, A.A., Maarouf, A.A.A., Abd El Al, S.A., Fatma, I.E.H., El Mougy, E.E.A., 2014. Detection of some virulence genes of avian pathogenic E.coli by polymerase chain reaction. Benha Vet. Medical J. 26: 159-176.
Ammar, A.M., Norhan, K.A., Yousreya, H.M., Abd El- Aziz, E.E., 2011. Advanced studies on Diagnosis of Single M. gallisepticum infection and combined with E.coli in chickens. Zag.Vet. J. 79: 110-114.
Arciola, C.R., Campoccia, D., Gamberini, S., Baldassarri, L., Montanaro, L., 2005. Prevalence of cna fnbA and fnbB adhesin genes among Staphylococcus aureus isolates from orthopedic infections associated to different types of implant. FEMS microbiology letters 246: 81-86.
Bkheet, A.A., 2011 bacterial aspects of arthiritis in Broiler chickens. Zagazig Vet. J. 39: 158-165.
Bradbury, J., Yavari, C., Dare, C., 2001. Detection of Mycoplasma synoviae in clinically normal pheasants. The Vet. Record 148: 72-74.
Chansiripornchai, V.R.N., 2009. The efficacy of Escherichia coli aroA-live vaccine in broilers against avian E. coli serotype O78 infection. Thai J. Vet. Med 39, 337-342.
Cunha, M.P.V., de Oliveira, M.G.X., de Oliveira, M.C.V., da Silva, K.C., Gomes, C.R., Moreno, A.M., Knöbl, T., 2014. Virulence profiles, phylogenetic background, and antibiotic resistance of Escherichia coli isolated from Turkeys with Airsacculitis. The Sci. World J. 2014.
Germon, P., Chen, Y.-H., He, L., Blanco, J.E., Brée, A., Schouler, C., Huang, S.-H., Moulin-Schouleur, M., 2005. ibeA, a virulence factor of avian pathogenic Escherichia coli. Microbiol. 15: 1179-1186.
Ghafouri, S., Bozorgmehrifard, M., Karimi, V., Nazemshirazi, M., Noormohammadi, A., Hosseini, H., 2011. Identification and primary differentiation of Iranian isolates of Mycoplasma synoviae using PCR based on amplification of conserved 5′ end of vlhA gene. J. Vet. Res. 66:117-122.
Gu, C.-Q., Hu, X.-Y., Xie, C.-q., Zhang, W.-P., Wang, D.-H., Zhou, Q., Cheng, G.-F. 2013. Observation on arthritis in broiler breeder chickens experimentally infected with Staphylococcus aureus. Mortality 100: 10.
Hu, Q., Tu, J., Han, X., Zhu, Y., Ding, C., Yu, S., 2011. Development of multiplex PCR assay for rapid detection of Riemerella anatipestifer, Escherichia coli, and Salmonella enterica simultaneously from ducks. J. Microbiol. Methods 87:64-69.
Huff, G., Huff, W., Rath, N., Balog, J., 2000. Turkey osteomyelitis complex. Poult. Sci. 79: 1050-1056.
Jeffery, N., Gasser, R.B., Steer, P.A., Noormohammadi, A.H., 2007. Classification of Mycoplasma synoviae strains using single-strand conformation polymorphism and high-resolution melting-curve analysis of the vlhA gene single-copy region. Microbiol. 153: 2679-2688.
Jeong, Y.-W., Kim, T.-E., Kim, J.-H., Kwon, H.-J., 2012. Pathotyping avian pathogenic Escherichia coli strains in Korea. J. Vet. Sci. 13: 145-152.
Johnson, J.R., Stell, A.L., 2000. Extended virulence genotypes of Escherichia coli strains from patients with urosepsis in relation to phylogeny and host compromise. J. Infectious Dis. 181: 261-272.
Josefsson, E., Hartford, O., O’Brien, L., Patti, J.M., Foster, T., 2001. Protection against experimental Staphylococcus aureus arthritis by vaccination with clumping factor A, a novel virulence determinant. J. Infect.Dis. 184: 1572-1580.
Kleven, S.H., 1997. Mycoplasma synoviae infection. In: Diseases of Poultry. (Calnek BW, Barnes HJ, Beard CW, McDougald LR and YM Saif; eds). Iowa State University Press, Ames, Iowa, USA, pp: 220–228.
Ley, D.H., Saify, M., Barnes, H.J., Glisson, J.R., Fadley, A.M., McDougaldl, R., Swayne, D.E., 2003. Mycoplasma synoviaeinfection.Disease of poultry, EleventhEdition.Iowa State University press, Iowa, USA, 756-766.
Mamza, S.A., Egwu, G.O., Mshelia, G.D., 2010. Antibiotic susceptibility patterns of beta-lactamase-producing Escherichia coli and Staphylococcus aureus isolated from chickens in Maiduguri (Arid zone), Nigeria. Veterinarski Arhiv 80: 283-297.
Mason, W.J., Blevins, J.S., Beenken, K., Wibowo, N., Ojha, N., Smeltzer, M.S., 2001. Multiplex PCR protocol for the diagnosis of staphylococcal infection. J. Clin. Microbiol. 39: 3332-3338.
McNamee, P.T., Smyth, J.A., 2000. Bacterial chondronecrosis with osteomyelitis ('femoral head necrosis') of broiler chickens: a review. Avian Pathol. 29: 477-495.
Mohan, K., Shroeder-Tucker, L., Karenga, D., Dziva, F., Harrison, A., Muvavarirwa, P., 2002. Unidentified Coryneform Bacterial strain from cases of polyarthritis in Chickens: phenotype and fatty acid profile. Avian Dis. 46: 1051-1054.
Montanaro, L., Arciola, C.R., Baldassarri, L., Borsetti, E., 1999. Presence and expression of collagen adhesin gene (cna) and slime production in Staphylococcus aureus strains from orthopaedic prosthesis infections. Biomaterials 20: 1945-1949.
Morrow, C., Bell, I., Walker, S., Markham, P., Thorp, B., Whithear, K., 1990. Isolation of Mycoplasma synoviae from infectious synovitis of chickens. Australian Vet. J. 67: 121-124.
Paniagua-Contreras, G., Sáinz-Espu, T., Monroy-Pérez, E., Rodríguez-Moctezuma, J.R., Arenas-Aranda, D., Negrete-Abascal, E., Vaca, S., 2012. Virulence markers in Staphylococcus aureus strains isolated from hemodialysis catheters of Mexican patients.
Poveda, J., Carranza, J., Miranda, A., Garrido, A., Hermoso, M., Fernandez, A., Domenech, J., 1990. An epizootiological study of avian mycoplasmas in southern Spain. Avian Pathol. 19: 627-633.
Quinn, P.J., Markey, B.K., Leonard, F.C., Hartigan, P., Fanning, S., FitzPatrick, E.S., 2011. Vet. Microbiol. Microbial Dis. Wiley.
Rasheed, B., 2011. Isolation and identification of bacteria causing arthritis in chickens. Iraqi Journal of Veterinary Sciences 25, 93 (En)-95 (En).
Saif, Y.M., Fadly, AM., Glisson, J.R., McDougald, L.R., Nolan, L.K., Swayne, D.E., 2011. Dis. Poult.. Wiley.
Sambrook, J., Russell, D.W., 2001. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press.
Shaker, M.M., 1995. Microbiological studies on Mycoplasma infection in poultry.Ph.D. Thesis, Microbiology Dept., Fac. of Vet .Med. Cairo Univ. In, City.
Sullivan, T.W., 1994. Skeletal problems in poultry: estimated annual cost and descriptions. Poult. Sci. 73: 879-882.
Wang, Y., Tang, C., Yu, X., Xia, M., Yue, H., 2010. Distribution of serotypes and virulence-associated genes in pathogenic Escherichia coli isolated from ducks. Avian Pathol. 39: 297-302.
Wissman, M.A., Parsons, B., 1996. Mycoplasmosis in the common rhea (Rhea americana). J. Avian Med. Surg.15: 28-30.
Xu, Y., Rivas, J.M., Brown, E.L., Liang, X., Höök, M., 2004. Virulence potential of the staphylococcal adhesin CNA in experimental arthritis is determined by its affinity for collagen. J. Infect. Dis. 189: 2323-2333.