Maternal antibody decay and antibody-mediated immune responses in chicken pullets fed prebiotics and synbiotics
ABSTRACT
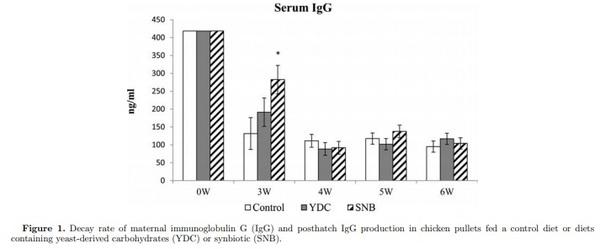
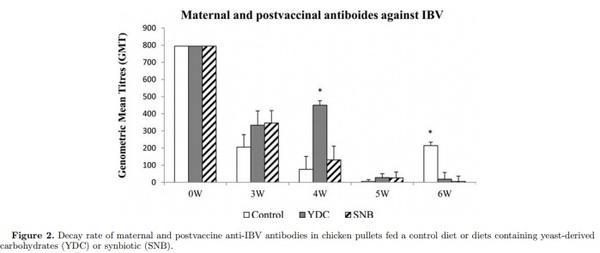
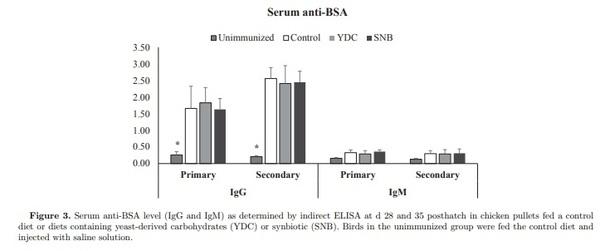
Three experiments were conducted to evaluate the effect of yeast-derived carbohydrates (YDC), and a blend of probiotics and YDC (synbiotic, SNB) on serum IgG concentration, maternal-derived antibody (MDA) decay, and specific antibody-mediated immune response in chick pullets following immunization with T-cell dependent antigens. A total of 300 day-old pullet chicks were randomly assigned to 3 dietary treatments including: a basal diet (Control), and diets containing YDC, and SNB (Lactobacillus acidophilus, L. casei, Streptococcus faecium, and Bacillus subtilis, and YDC). In experiment one, on d 1 and wk 3, 4, 5, and 6, blood samples were collected and serum were analyzed by ELISA for total IgG (Y), and MDA against Newcastle disease virus (NDV) and infectious bursal disease virus (IBDV). The second experiment examined the specific antibody against infectious bronchitis virus (IBV) in pullet chicks following vaccination against IBV at d 1. Finally, in experiment 3, on d 21 and 28 posthatch, 10 birds per treatment were immunized intramuscularly with both sheep red blood cells (SRBC) and bovine serum albumin (BSA), and 11 after immunization serum samples were analyzed by hemagglutination assay for antibody response to SRBC, and by ELISA for serum IgM and IgG response to BSA. The results demonstrated that diet containing SNB increased serum IgG at wk 3 posthatch. However, the decay rate of MDA against NDV and IBDV were not affected by dietary treatments. Birds fed YDC showed higher specific antibody response against IBV in wk 4, while both diets containing YDC and SNB decreased antibody response to IBV in wk 6. In addition, specific antibody response against SRBC and BSA was not affected by diets. In conclusion, supplementation of diet with SNB improved humoral immunity by increasing IgG concentration in serum, and modulated the adaptive antibody-mediated immune response against IBV.
Key words: probiotic, synbiotic, immunity, maternal, antibodies.
Ahmed, Z., and S. Akhter. 2003. Role of maternal antibodies in protection against infectious bursal disease in commercial broilers.
Int. J. Poult. Sci. 2:251–255.
Alizadeh, M., J. C. Rodriguez-Lecompte, H. Echeverry, G. H.
Crow, and B. A. Slominski 2016. Effect of yeast-derived products and distillers dried grains with solubles (DDGS) on
antibody-mediated immune response and gene expression of
pattern recognition receptors and cytokines in broiler chickens immunized with T-cell dependent antigens. Poult. Sci.
95:823–833.
Bar-Shira, E., D. Sklan, and A. Friedman. 2003. Establishment of
immune competence in the avian GALT during the immediate
posthatch period. Dev. Comp. Immunol. 27:147–157
Huyghebaert, G., R. Ducatelle, and F. Van Immerseel. 2011. An update on alternatives to antimicrobial growth promoters for broilers. Vet. J. 187:182–188.
Janardhana, V., M. Broadway, M. P. Bruce, J. W. Lowenthal, M. S.
Geier, R. J. Hughes, and A. G. D. Bean. 2009. Prebiotics modulate immune responses in the gut-associated lymphoid tissue of
chickens. J. Nutr. 139:1404–1409.
Jawhara, S., K. Habib, F. Maggiotto, G. Pignede, P. Vandekerckove, E. Maes, L. Dubuquoy, T. Fontaine, Y. Guerardel, and D.
Poulain. 2012. Modulation of intestinal inflammation by yeasts
and cell wall extracts: strain dependence and unexpected antiinflammatory role of glucan fractions. PLoS One. 7:e40648.
Klasing, K. C. 2007. Nutrition and the immune system. Br. Poult.
Sci. 48:525–537.
Kramer, T., and H. Cho. 1970. Transfer of immunoglobulins and
antibodies in the hen’s egg. Immunology. 19:157–167.
Larsson, A., R. -M. Balow, T. L. Lindahl, and P. -O. Forsberg.
1993. Chicken antibodies: taking advantage of evolution-a review.
Poult. Sci. 72:1807–1812.
Lebeer, Sarah, Jos Vanderleyden, and Sigrid C. J. De Keersmaecker.
2010. Host interactions of probiotic bacterial surface molecules:
comparison with commensals and pathogens. Nature Rev. Microbiol. 8:171–184.
McCracken, V., H. Gaskins, and G. Tannock. 1999. Probiotics and
the immune system. Pages 85–111 in: G. Tannock, ed., Probiotics:
A Critical Review. Horizon Scientific Press, Helsinki, Finland.
McEwen, S. A., and P. J. Fedorka-Cray. 2002. Antimicrobial use and
resistance in animals. Clin. Infect. Dis. 34:S93–S106.
Midilli, M., M. Alp, and N. Kocabach. 2008. Effects of dietary probiotic and prebiotic supplementation on growth performance and
serum IgG concentration of broilers. S. Afr. J. Anim. Sci. 38:21–
27.
Mondal, S., and S. Naqi. 2001. Maternal antibody to infectious
bronchitis virus: its role in protection against infection and development of active immunity to vaccine. Vet. Immunol. Immunopathol. 79:31–40.
Naqi, S., B. Marquez, and N. Sahin. 1983. Maternal antibody and
its effect on infectious bursal disease immunization. Avian Dis.
27:623–631.
Obukhanych, T. V., and M. C. Nussenzweig. 2006. T-independent
type II immune responses generate memory B cells. J. Exp. Med.
203:305–310.
Panda, A. K., S. V. R. Rao, M. V. Raju, and S. R. Sharma. 2006.
Dietary supplementation of Lactobacillus sporogenes on performance and serum biochemico-lipid profile of broiler chickens.
Poult. Sci. 43:235–240.
Brommer, J. E. 2004. Immunocompetence and its costs during development: an experimental study in blue tit nestlings. R. Soc.
Lond. B. Biol. Sci. 271:110–113.
Canadian Council on Animal Care. 1993. Guide to the Care and Use
of Experimental Animals 1. 2nd ed. Canadian Council on Animal
Care.
Cerutti, A., and M. Rescigno. 2008. The biology of intestinal immunoglobulin A responses. Immunity. 28:740–750.
Christensen, H. R., H. Frokiær, and J. J. Pestka. 2002. Lactobacilli
differentially modulate expression of cytokines and maturation
surface markers in murine dendritic cells. J. Immunol. 168:171–
178.
Clavel, T., and D. Haller. 2007. Molecular interactions between bacteria, the epithelium, and the mucosal immune system in the intestinal tract: implications for chronic inflammation. Curr. Issues.
Intest. Microbiol. 8:25–43.
De Vries, P., I. Visser, J. Groen, H. Broeders, F. Uytdehaag, and
A. Osterhaus. 1990. Immunogenicity of measles virus iscoms in
the presence of passively transferred MV-specific antibodies. Vaccines. 90:139–144.
Dibaji, S. M., A. Seidavi, L. Asadpour, and F. M. da Silva. 2014.
Effect of a synbiotic on the intestinal microflora of chickens. J.
Appl. Poult. Res. 23:1–6.
Dibner, J., and J. Richards. 2005. Antibiotic growth promoters in
agriculture: history and mode of action. Poult. Sci. 84:634–643.
Fink, K., R. Zellweger, J. Weber, N. Manjarrez-Orduno, M. Holdener, B. M. Senn, H. Hengartner, R. M. Zinkernagel, and A. J.
Macpherson. 2008. Long-term maternal imprinting of the specific
B cell repertoire by maternal antibodies. Eur. J. Immunol. 38:90–
101.
Ganan, M., J. M. Silv´an, A. V. Carrascosa, and A. J. Mart´inezRodr´iguez. 2012. Alternative strategies to use antibiotics or chemical products for controlling Campylobacter in the food chain.
Food. Control. 24:6–14.
Gasparini, J., K. D. McCoy, V. Staszewski, C. Haussy, and T.
Boulinier. 2006. Dynamics of anti-Borrelia antibodies in Blacklegged Kittiwake (Rissa tridactyla) chicks suggest a maternal educational effect. Can. J. Zool. 84:623–627.
Ghosh, T., S. Haldar, M. Bedford, N. Muthusami, and I. Samanta.
2012. Assessment of yeast cell wall as replacements for antibiotic
growth promoters in broiler diets: effects on performance, intestinal histomorphology and humoral immune responses. J. Anim.
Physiol. Anim. Nutr. 96:275–284.
Grindstaff, J. L. 2008 Maternal antibodies reduce costs of an immune
response during development. J. Exp. Biol. 211:654–660.
Grindstaff, J. L., D. Hasselquist, J. -°A. Nilsson, M. Sandell,
H. G. Smith, and M. Stjernman. 2006. Transgenerational
priming of immunity: maternal exposure to a bacterial antigen enhances offspring humoral immunity. Proc. R. Soc.
273:2551–2557.
Guo, Y., R. Ali, and M. Qureshi. 2003. The influence of β-glucan on
immune responses in broiler chicks. Immunopharmacol. Immunotoxicol. 25:461–472.
Haghighi, H., J. Gong, C. L. Gyles, M. A. Hayes, B. Sanei, P. Parvizi,
H. Gisavi, J. R. Chambers, and S. Sharif. 2005. Modulation of
antibody-mediated immune response by probiotics in chickens.
Clin. Diagn. Lab. Immunol. 12:1387–1392.
Haghighi, H., J. Gong, C. L. Gyles, M. A. Hayes, H. Zhou, B. Sanei,
J. R. Chambers, and S. Sharif. 2006. Probiotics stimulate production of natural antibodies in chickens. Clin. Vaccine Immunol.
13:975–980.
Hamal, K. R., S. Burgess, I. Pevzner, and G. Erf. 2006. Maternal
antibody transfer from dams to their egg yolks, egg whites, and
chicks in meat lines of chickens. Poult. Sci. 85:1364–1372.
Heller, E., H. Leitner, N. Drabkin, and D. Melamed. 1990. Passive
immunisation of chicks against Escherichia coli. Avian. Pathol.
19:345–354.
Hooper, L. V., D. R. Littman, and A. J. Macpherson. 2012. Interactions between the microbiota and the immune system. Science.
336:1268–1273.
Huang, M., Y. Choi, R. Houde, J. Lee, B. Lee, and X. Zhao. 2004. Effects of Lactobacilli and an acidophilic fungus on the production
performance and immune responses in broiler chickens. Poult. Sci.
83:788–795.
Parker, D. C. 1993. T cell-dependent B cell activation. Annu. Rev.
Immunol. 11:331–360.
Sahin, O., Q. Zhang, J. Meitzler, B. Harr, T. Morishita, and R.
Mohan. 2001. Prevalence, antigenic specificity, and bactericidal
activity of poultry anti-Campylobacter maternal antibodies. Appl.
Environ. Microbiol. 67:3951–3957.
Scott, T. 2004. Our current understanding of humoral immunity of
poultry. Poult. Sci. 83:574–579.
Sharma, J. M. 1997. The structure and function of the avian immune
system. Acta. Vet. Hung. 45:229–238.
Shashidhara, R., and G. Devegowda. 2003. Effect of dietary mannan
oligosaccharide on broiler breeder production traits and immunity. Poult. Sci. 82:1319–1325.
Siegrist, C. -A., M. C´ordova, C. Brandt, C. Barrios, M. Berney, C.
Tougne, J. Kovarik, and P. -H. Lambert. 1998. Determinants of
infant responses to vaccines in presence of maternal antibodies.
Vaccine. 16:1409–1414.
Soler, J. J., L. de Neve, T. P´erez–Contreras, M. Soler, and G. Sorci.
2003. Trade-off between immunocompetence and growth in magpies: an experimental study. R. Soc. Lond. B. Biol. Sci. 270:241–
248.
Sugita-Konishi, Y., K. Shibata, S. S. Yun, Y. Hara-Kudo, K.
Yamaguchi, and S. Kumagai. 1996. Immune functions of immunoglobulin Y isolated from egg yolk of hens immunized
with various infectious bacteria. Biosci. Biotechnol. Biochem.
60:886–888.
Talebi, A., B. Amirzadeh, B. Mokhtari, and H. Gahri. 2008. Effects
of a multi-strain probiotic (PrimaLac) on performance and antibody responses to Newcastle disease virus and infectious bursal disease virus vaccination in broiler chickens. Avian. Pathol.
37:509–512.
Van Binnendijk, R. S., M. C. Poelen, G. van Amerongen, P. de Vries,
and A. D. Osterhaus. 1997. Protective immunity in macaques vaccinated with live attenuated, recombinant, and subunit measles
vaccines in the presence of passively acquired antibodies. J. Infect. Dis. 175:524–532.
Wegener, H. C. 2003. Antibiotics in animal feed and their role in
resistance development. Curr. Opin. Microbiol. 6:439–445.
Yang, Y., P. Iji, and M. Choct. 2009. Dietary modulation of gut
microflora in broiler chickens: a review of the role of six kinds of
alternatives to in-feed antibiotics. World’s Poult. Sci. J. 65:97–
114.
Yitbarek, A., H. Echeverry, P. Munyaka, and J. C. RogriguezLecompte. 2015. Innate immune response of pullets fed diets supplemented with prebiotics and synbiotics. Poult. Sci. 94:1802–
1811.










.jpg&w=3840&q=75)