Mannan oligosaccharides in poultry nutrition
Mannan oligosaccharides in poultry nutrition: mechanisms and benefits
Introduction
Mannan oligosaccharide (MOS) is derived from the cell wall of Saccharomyces cerevisiae and is commercially available as a feed supplement included in diets as a GRAS (generally regarded as safe) compound. The benefits of MOS are based on specific properties that include modification of the intestinal flora, reduction in turnover rate of the intestinal mucosa and modulation of the immune system in the intestinal lumen. These properties have the potential to enhance growth rate, feed conversion efficiency and livability in commercial broilers and turkeys and to increase egg production in breeders and table egg flocks.
Mechanisms underlying response to mannan oligosaccharides
INHIBITION OF PATHOGENS
Enteric pathogens attach to the enterocytes of the intestinal mucosa as a precursor to colonization. Adhesins are produced by fimbriae, which have an affinity for specific carbohydrate residues on the surface glycoproteins of the host cell. Bacteria with Type 1 fimbriae adhere specifically to mannose in the cell wall glycoprotein and are vulnerable to competitive inhibition. It is known that the lectins of bacterial fimbriae will agglutinate yeast cells containing mannose. This property has been evaluated for an extensive range of E. coli strains and Salmonella species using standard techniques (Spring et al., 2000). A commercial MOS product (Bio-Mos, Alltech Inc.) was used to evaluate agglutination by the E. coli and Salmonella cultures derived from commercial broiler and turkey flocks. Bio-Mos contains modified cell wall fragments of Saccharomyces cerevisiae, which are obtained by lysis followed by centrifugation and subsequent washing and spray drying. Bacteria possessing mannose-sensitive fimbriae agglutinate MOS. This reaction can be inhibited in the presence of mannose and the specificity of the adhesion can be evaluated against other sugars including fructose, galactose, and glucose. Generally, bacteria possessing Type 1 fimbriae that are agglutinated by MOS show mannose-specific attachment.
Although inhibition by fructose occurs frequently, other sugars do not prevent agglutination. In a survey of enteric pathogens, it was demonstrated that approximately 70% of 77 E. coli strains and 53% of 30 Salmonella species possessing Type 1 fibriae were sensitive to mannose (Finucane et al., 1999b; Table 1). Generally, Clostridia species and Campylobacter jejuni do not agglutinate MOS but individual isolates have been identified with weak agglutinating properties, independent of mannose inhibition, suggesting other mechanisms of bacterial adhesion associated with the cell wall extract of Saccharomyces cerevisiae.
Table 1. Incidence of seven bacterial genera possessing mannose-sensitive adhesins.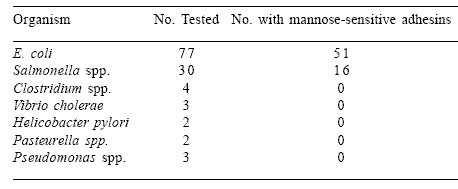
Subjecting MOS to a temperature of 120°C for 20 minutes did not affect the ability to adhere to mannose sensitive bacteria (Newman et al., 1995). It is therefore concluded that neither pelletization nor extrusion will inhibit the binding of MOS to intestinal pathogens sensitive to mannose in commercially processed feeds.
Inhibition of colonization with specific Salmonella and E. coli strains was clearly demonstrated in a series of trials using a standardized challenge technique (Spring et al., 2000). In these studies, chicks were hatched from eggs decontaminated by immersion in a disinfectant solution and then incubated in a sterile setter-hatcher combination located in an isolation chamber. Day-old chicks were infected with a Salmonella-free standard culture derived from hatching debris which incorporated coliforms, enterococci and lactobacilli. Chicks, housed in bacterial isolators, were challenged with the test organisms including Salmonella, E. coli and Campylobacter; and colonization of the ceca was determined following sacrifice at 10 days of age.
Inclusion of MOS in feed at 4 g/kg decreased the prevalence of colonization with Salmonella dublin in three consecutive trials from 89.8% to 55.7%. This difference was statistically significant (P< 0.05). The concentration of Salmonella typhimurium strain 29 E was significantly reduced in three consecutive trials from 5.40 to 4.01 log CFU/g cecal contents. The concentrations of coliforms, lactobacilli and anaerobes were unaffected by inclusion of MOS in diets. The inhibition of Salmonella colonization was not associated with any change in cecal pH or elevation in propionic acid concentration suggesting a specific interaction between MOS and Salmonella. A similar reduction in the concentration of S. typhimurium from 6.28 to 4.13 log CFU/g of cecal contents was demonstrated by Belamaranahally (2000) applying standard procedures for incubation of eggs, housing of chicks and enumeration of cecal Salmonella (Figure 1).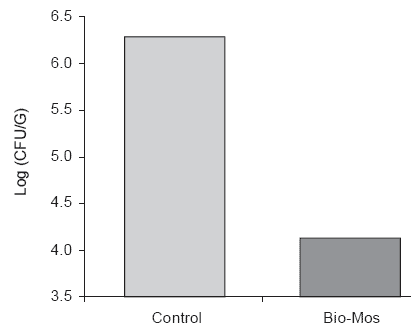
Figure 1. Effect of dietary Bio-Mos on cecal S. typhimurium 29E concentrations of
chicks 7 days after Salmonella challenge (Belamaranahally, 2000).
The specificity of individual strains of E. coli with respect to sensitivity to MOS is indicated by the results of a cooperative trial conducted in Switzerland and the USA (Spring et al., 1996). Although six successive trials showed no significant difference in the concentration of total cecal coliforms, E. coli strain 15R, known to possess Type 1 fimbriae, was reduced in prevalence from 75% of chicks challenged with this organism to 15% in the treatment receiving dietary Bio-Mos at a level of 4 g/kg. A statistically significant reduction in S. dublin was observed, from 89.8% of challenged chicks in the control group to 55.8% in the treatment receiving dietary MOS.
The quantum of Clostridium species in the cecal contents of 6 week old turkey poults was significantly reduced by inclusion of MOS in the diet at a level of 1 g/kg. In a replicate pen trial, total counts of Clostridium spp.
were significantly reduced from 4.2 to 2.8 log CFU/g cecal contents. This effect did not persist through the growing period. At 18 weeks of age there was no significant difference in the level of Clostridium spp. in cecal contents (Finucane et al., 1999a; Figure 2).
Mannan oligosaccharides in poultry nutrition: mechanisms and benefits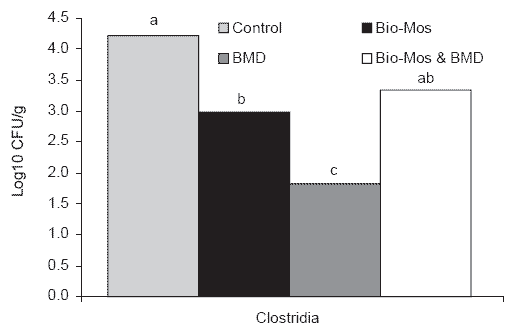
Figure 2. Concentrations (log10 CFU/g) of clostridia in the large intestines of turkeys fed Bio-Mos, bacitracin methylene disalicylate (BMD), or Bio-Mos with BMD (Period 1, 6 weeks) (a,b,cMeans differ, P<0.05).
CHANGES IN MORPHOLOGY OF THE INTESTINAL TRACT
There is substantial evidence that dietary MOS modifies the morphology and structure of the intestinal mucosa. Enterocytes undergo a continual cycle of proliferation in the intestinal crypt, maturation and migration up the villi with desquamation at the tip of the villus. The depth of the crypt is a function of the rate of cell replacement. The process can be accelerated by ingesting toxins, or production of deleterious compounds including ammonia by the intestinal flora. Accelerated replacement of enterocytes requires diversion of energy and protein from growth and the development of tissues and organ systems. Studies conducted at Oregon State University demonstrated a decrease in the depth of the intestinal crypts in turkey poults through 8 weeks of age in three sections of the intestine comprising the distal half of the duodenal loop; Meckel’s diverticulum and at the junction of the jejunum and cecum (Savage et al., 1997). The depth of the crypts was significantly reduced by inclusion of 1% MOS in diets in comparison to the controls (Table 2). There was no change in the number of goblet cells in the mucosa of the villi as a result of including MOS in any of the three regions of the intestine examined. The changes in intestinal morphology were correlated to a statistically significant increase in growth rate through eight weeks of age, suggesting an inverse correlation between the parameters measured.
Table 2. Effect of Bio-Mos inclusion level on goblet cell numbers and crypt depth at different points in the intestine*.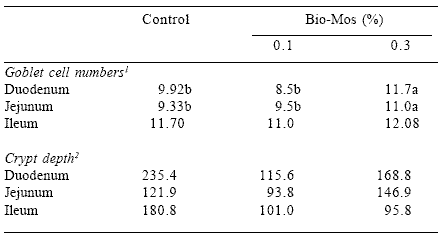
*Savage et al., 1997.
1 counted along 250 μm of the villus tip (125 μm, each side).
2 Crypt depth was calculated as the difference between villus length and villus height. Villus length
was determined as the distance from the villus tip to muscular mucosa in mm. Villus height was
determined as the distance from villus tip to the villus base, in μm. All distances were determined
using a calibrated ocular micrometer.
MODULATION OF IMMUNE RESPONSE
Various experiments have been conducted to examine the effect of MOS on humoral and cell immunity. Studies conducted at Ohio State University in conjunction with Framingham State College in Massachusetts
demonstrated that dietary Bio-Mos at 0.5 and 1 g/kg inclusion enhance the serum antibody titer against sheep erythrocyte antigen administered one week prior to evaluation using a microtiter procedure (Lilburn et al., 2000). The stimulatory effect of MOS on antibody titer was statistically significant seven days after sensitization and numerical differences persisted through the following four weeks as titers waned (Figure 3).
Inclusion of MOS at 1 g/kg diet enhanced both IgG and IgA serum antibody levels in turkey poults (Savage et al., 1996; Figure 4). Statistically significant increases in both immunoglobulins were detected at 7 ½ weeks
of age. The increased systemic response by the immune system was correlated with statistically significant improvement in growth rate of the poults without any effect on feed conversion efficiency, although numerical differences were observed in favor of supplementation with MOS.
The effect of MOS at the cell level is demonstrated by stimulation of phagocytosis in vitro. Luminol-enhanced chemiluminescence was applied to demonstrate the activity of phagocytes in the presence of incremented
levels of dietary MOS. A dose response was measured by chemiluminescence with values increasing from 1 to 1.8 mV over a range of MOS concentrations extending from 0 through 2000 μg/sample preparation (Sisak,
1995; Figure 5).
Mannan oligosaccharides in poultry nutrition: mechanisms and benefits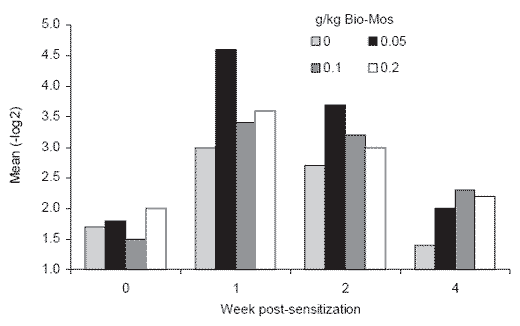
Figure 3.Effect of Bio-Mos level in a commercial layer diet on serum antibody titer against sheep erythrocyte antigen (Lilburn et al., 2000)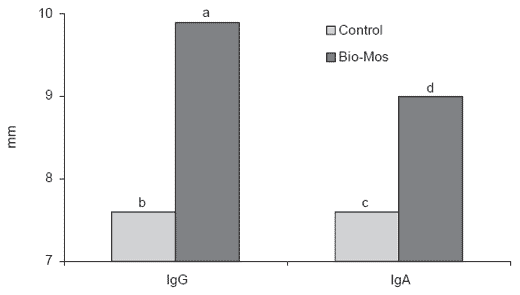
Figure 4.Quantification (height of the precipitin arc in mm) of plasma IgG and bile IgA using rocket-immunoelectrophoresis of Wrolstad Medium White male poults (abP<0.001, cdP<0.007) (Savage et al., 1996).
Inclusion of MOS in the diets of replacement pullets at a level of 1 g/kg reduced the intensity of the delayed wattle hypersensitivity reaction (Cotter and Weinner, 1997). Injection of a phytohemagglutin into the wattle induces a severe swelling after approximately three hours, which persists for up to four days. The thickness of the wattle is a direct measure of the inflammatory response to the antigen. A significant reduction was recorded in both 8 and 10-week-old pullets subjected to three successive exposures to the antigen at 2-week intervals commencing at the 6th week (Table 3). 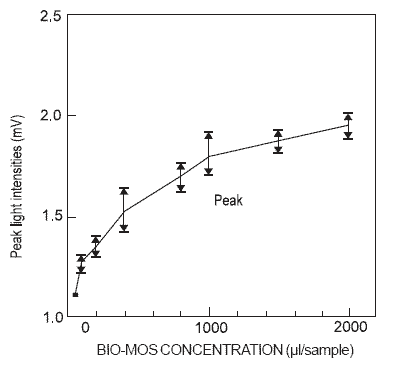
Figure 5.Luminol-enhanced chemiluminescense of rat phagocytes activated with increasing concentrations of mannan oligosaccharide (Bio-Mos).
The mechanism of dietary MOS in reducing the inflammatory tissue response is unknown but may be associated with binding and inactivation of the antigen directly involved in the hypersensitivity reaction by modulating one or more of the compounds in the cascade of cytokines.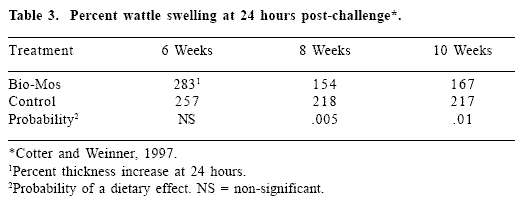
Performance responses to mannan oligosaccharides
The effect of dietary MOS supplementation on the integrity and structure of the intestinal mucosa, impact on the composition of the microflora including pathogens and the indirect influence on the immune system at both the systemic and cellular levels produces a beneficial response in field performance in both broilers and turkeys. The value of a feed additive is measured by the benefit to cost ratio, derived from the incremental revenue and the cost of supplementation. A number of controlled pen studies and field trials have been conducted to establish the response to MOS. The performance promoting effect has assumed increasing importance during the past three years following the ban on growth stimulating antibiotics in the EU, and similar trends in industrialized nations with large export markets or with domestic demand for high value, ‘organic’ foods.
BROILERS
A positive response in body weight and feed conversion efficiency was documented in trials conducted in the Czech Republic with cage-housed commercial broiler chicks (Kumprecht et al., 1997). A statistically significant
increase in live weight was noted in all treatments receiving dietary Bio-Mos at levels of 0.5 through 3 g/kg at 21 days. Feed conversion efficiency was improved through the 42 day experimental period. The protocol also
included measurement of nutrient digestibility. All levels of dietary MOS enhanced the utilization of crude fiber from 6.23% in controls to a mean of 12.8%. This is attributed to an alteration in the flora of the cecum and the distal intestinal tract.
During the past four years, a number of trials have been conducted contrasting MOS with conventional antibiotic growth stimulants. A replicate pen trial conducted by Virginia Scientific Research, Inc. contrasted
unsupplemented control diets with Bio-Mos at 1 g/kg in the starter and 0.5 g/kg in the grower and finisher diets (Sims and Sefton, 1999; Table 4). This was compared to bacitracin at 50 g/ton in the starter and 25 g/ton in the finisher diets, respectively. Live weight and feed conversion efficiency were both statistically enhanced compared to the unsupplemented control at 49 days of age. Liveability, which ranged from 3.5 to 5.8%, was unaffected by treatment. Both Bio-Mos and bacitracin yielded significantly higher revenue in terms of the mass of processed carcasses sold and a more favorable return over feed cost compared to the unsupplemented control. The numerical advantage of bacitracin over Bio-Mos was $US 0.03/bird after feed costs, but there was no significant difference between the two additives with respect to incremental revenue. The study demonstrated that MOS, a non-antibiotic additive, was equivalent to bacitracin with respect to technical performance and financial return.
In the EU, only avilamycin and bambermycins are available as approved antibiotic growth stimulants. An extensive trial was conducted by a large integrator in the UK to compare the performance of flocks receiving Bio-Mos compared to avilamycin (Kenyon, personal communication). Three flocks were placed sequentially during the period April to September 1999 and standard commercial diets and management procedures were used.
Table 4.Effects of Bio-Mos, BMD and a negative control on broiler performance*.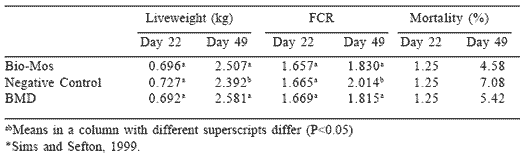
Based on the favorable performance of the three flocks receiving MOS, which were statistically similar in live weight, conversion and livability, the integrator substituted MOS for the approved antibiotic growth stimulant
and thereby qualified the poultry meat produced for ‘antibiotic-free’ status, which generated a higher revenue in the premium market (Table 5).
Table 5.Comparison of Bio-Mos and avilamycin on performance of commercial broilers in
Britain.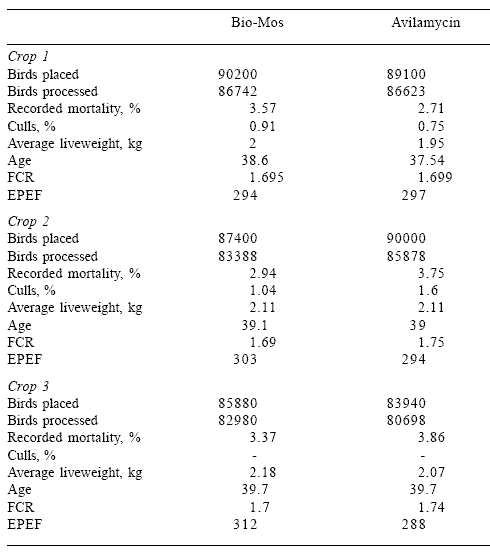
In a trial conducted in Canada at St. Hyacinthe in Quebec, a statistically significant improvement in 42-day live weight was obtained in commercial high-yield broilers aged 42 days (Figure 6). MOS was included at 1 g/kg in the starter and 0.5 g/kg in the finisher. This treatment was statistically superior to supplementation with bambermycins at 0.5 g/kg. There were no statistically significant differences in either feed intake or feed conversion efficiency between MOS and bambermycins and both additives were superior to a non-supplemented control.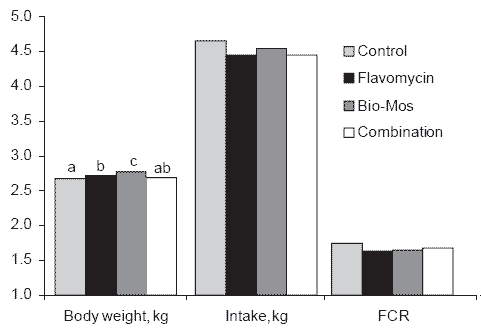
Figure 6.Comparative effects of Bio-Mos and Flavomycin on broiler performance (a,b,c
Means differ, P<0.05).
A second trial conducted in western Canada compared the performance of broilers receiving diets comprising wheat and barley as the cereal components and soybean meal and meat meal as the protein sources. Bio-Mos was incorporated in the starter and finisher diets at 2 g/kg and 1 g/kg, respectively.
This treatment was compared to dietary inclusion of virginiamycin at 50 ppm. Since there was no replication in this commercial field trial, statistical analysis was not possible but a numerical advantage was demonstrated for Bio-Mos with respect to live weight, feed conversion efficiency, production efficiency index, and feed cost per kg live weight.
TURKEYS
Both cost factors and restraints on the use of antibiotic growth promotors have stimulated evaluation of MOS supplementation of diets for commercial turkeys. A study conducted by AFSSA in France demonstrated the beneficial effect of MOS (2 g/kg 0 to 4 weeks: 1 g/kg 4 to 8 weeks) compared to either dietary avilamycin at 10 ppm or lactic acid added to water at a level of 1 g/l. Ingredients in the basal diet included maize, wheat, soybean meal, full fat soybeans, lupin meal, amino acids and a mineral supplement. Through 8 weeks of age, MOS contributed to significantly higher live weight compared to the antibiotic additive and both were superior to either the lactic acid treatment or the controls, which were not significantly different from each other (Table 6). By 16 weeks of age, there was no significant difference among the control, lactic acid and MOS treatments with a statistically significant superiority for avilamycin. There were no significant differences in feed or water intake, feed conversion efficiency or livability at 16 weeks of age. The trial demonstrated the beneficial effect of both MOS and avilamycin, especially in the starter period through 8 weeks of age, with respect to the body weight of toms.
Table 6.Comparative effects of avilamycin, lactic acid and Bio-Mos on performance of tom
turkeys*.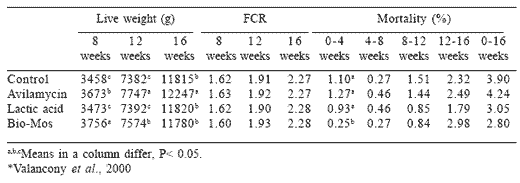
A replicate pen trial conducted in Virginia compared the effect of Bio-Mos and bacitracin alone and in combination, on the performance of tom turkeys raised through 18 weeks (Sims et al., 1999). To replicate field exposure to pathogens, used litter was spread in each pen, which was layered with fresh pine shavings. The respective inclusion levels of additives were bacitracin at 55 ppm in the starter and 27.5 ppm to finish and Bio-Mos at 1 g/kg in the starter, 0.5 g/kg to finish. The combination treatment included both of the additives at the indicated levels. No significant differences in live weight were noted through 12 weeks. At 18 weeks of age, both the Bio-Mos and bacitracin treatments were statistically superior to the control.
The combination yielded a higher live weight than either of the individual additives. Improved feed conversion efficiency was recorded in the treatments receiving the additives from week 12 onward compared to the
controls. The enhanced weight gain and feed conversion efficiency attributed to the additives lowered feed cost per bird and per kg of processed meat with no significant difference between the additives or their combination compared to the control. It was concluded that Bio-Mos was a practical and financially acceptable alternative to bacitracin in markets where antibiotic growth stimulants are either banned or are associated with market rejection.
In a field trial conducted in Minnesota, poults receiving MOS (2 g/kg through 3 weeks followed by 1 g/kg to 18 weeks) showed numerically superior livability, live weight, feed conversion efficiency and plant quality compared to a flock receiving bacitracin at the conventional level of 55 ppm in the starter followed by 27.5 ppm in subsequent diets through to depletion.
Conclusions
Supplementation of poultry diets with commercially available MOS enhances profitability by improving live bird performance parameters. Field trials have demonstrated superior growth rate and feed conversion efficiency, which relate directly to enhanced margins over feed and chick costs. The benefits are attributed to diverse mechanisms that are associated with modifying the composition of the intestinal microflora and modulation of the immune system.
Quantifying improvements from a feed supplement under field conditions is complicated by inadvertent bias in the selection of houses and flocks for comparison. Results may be confounded by climatic factors, disease challenge, variation in ingredient quality and dietary composition, management factors and their mutual interaction. Controlled replicate pen and cage trials that allow statistical analysis of data have confirmed the benefits of MOS as a feed additive in both broiler chicks and turkey poults. The advantages accruing to integrators and farmers will vary according to their cost structure, prevailing disease challenge and the range of ingredients available. It is evident that producers should conduct appropriately structured trials to evaluate the potential return derived from supplementing diets with MOS.
It is fortunate that MOS, an acceptable ‘natural’ substitute for antibiotic growth stimulants is commercially available for the poultry industry. Legislative bans on the use of antibiotics will intensify due to growing awareness among consumers and the scientific community of the dangers of emerging drug resistance.
References
Belamaranahally, V. 2000. Mannanoligosaccharides: Effects on cecal colonization by S. typhimurium. Poster presented at the 16th Annual Symposium on Biotechnology in the Feed Industry, Lexington, KY. May 3-5.
Cotter, P.F. and J. Weinner. 1997. Dietary Bio-Mos modulates kinetics of the phytohemagglutin wattle reaction in chickens. Poultry Sci. 76(Suppl. 1):111.
Finucane, M.C., Dawson, K.A., Spring, P. and K.E. Newman. 1999a. Effects of mannanoligosaccharide on composition of the gut microflora of turkey poults. Poultry Sci. 78(Suppl. 1):77.
Finucane, M., Spring, P. and K.E. Newman. 1999b. Incidence of mannosesensitive adhesions in enteric bacteria. Poultry Sci. 78(Suppl.1):139.
Kumprecht, I., Zobac, P., Siske, V., Sefton, A.E. and P. Spring. 1997. Effects of dietary mannanoligosaccharide level on performance and nutrient utilization of broilers. Poultry Sci. 76(Suppl. 1):132.
Lilburn, M., Dixon, J., Cotter, P., Paluch, B., Malzone, A., Sefton, T. and A. Connolly. 2000. Modulation of humoral immunity in commercial laying hens by a dietary probiotic. Poultry Sci. 79(Suppl. 1):38.
Newman, K.E., Spring, P. and L.S. Snitzer. 1995. Effect of thermal treatment on the ability of mannan oligosaccharide to adsorb enteric bacteria. J Anim. Sci. 73(Suppl. 1):310.
Savage, T.F., Cotter, P.F. and E.I. Zakrzewska. 1996. The effect of feeding a mannanoligosaccharide on immunoglobulin plasma IgA and bile IgA of Wrolstad MW male turkeys. Poultry Sci. 75(Suppl. 1):143.
Savage, T.F., Zakrzewska, E.I. and J.R. Andreasen. 1997. The effects of feeding mannan oligosaccharide supplemented diets to poults on performance and the morphology of the small intestine. Poultry Sci. 76(Suppl. 1):139.
Sims, M.D., White, M.F., Alexander, T.W., Sefton, T., Connolly, A. and P. Spring. 1999. Evaluation of Bio-Mos® fed alone and in combination with BMD to growing tom turkeys. Poultry Sci. 78(Suppl. 1):105.
Sims, M.D. and A.E. Sefton. 1999. Comparative effects of a mannan oligosaccharide and an antibiotic growth promoter on performance of commercial broilers. Proceedings of the 50th North Central Avian Disease
Conference, pg.
Sisak, F. 1995. Bio-Mos-mediated stimulation of phagocytosis as assessed by luminol-enhanced chemiluminescence. Czech Research Institute. Poster presented at the 11th Annual Symposium on Biotechnology in the Feed Industry. Lexington, KY. April.
Spring, P., Dawson, K.A., Wenk, C. and K.E. Newman. 1996. Effect of mannanoligosaccharide on different cecal parameters and on cecal concentration of enteric bacteria in challenged broiler chicks. Poultry Sci. 75(Suppl. 1):146.
Spring, P., Wenk, C., Dawson, K.A. and K.E. Newman. 2000. The effects of dietary mannanoligosaccharides on cecal parameters and the concentrations of enteric bacteria in the ceca of Salmonella-challenged broiler chicks. Poultry Sci. 79:205-211.
Valancony, H., M. Bougon; L. Balaire and P. Drouin. 2000. Impact of Bio-Mos and Avilamycin in Feed and Lactic Acid in Drinking Water on Performance of Tom Turkeys. Agence Francaise de Sécurité Sanitaire et Alimentaire (AFSSA), Plougragan, France.








