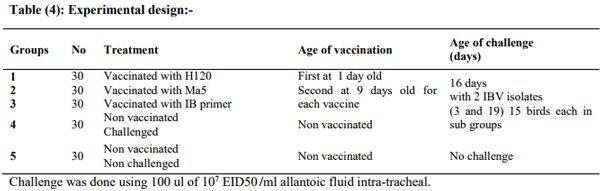
Incidence of Avian Nephritis and Infectious Bronchitis Viruses in Broilers in Egypt
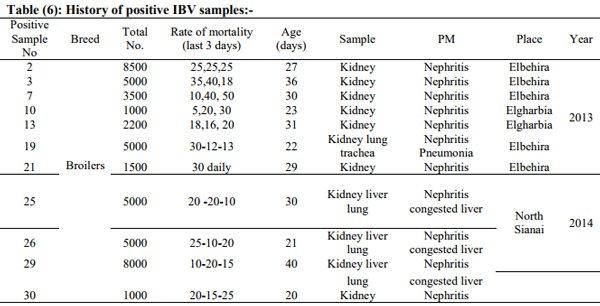
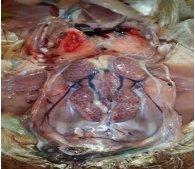
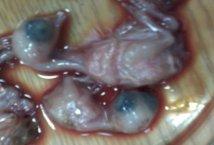
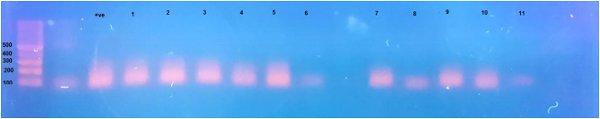
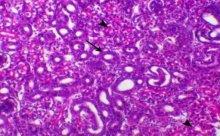
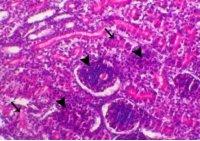
Avian nephritis virus is a new circulating virus in Europe, Australia, and Japan, this virus lead to growth retardation of young chickens by causing interstitial nephritis. Investigation was done in 5 Egyptian governorates (North Sinai, El-Minoufiya, ElGharbia and El-Behera and Kafr-Elsheikh) on 28 broiler chicken flocks and all the flocks were negative for Avian nephritis virus. In addition, we continued the detection of IBV in the same 28 broiler chicken flocks, resulting that 11 flocks out of 28 flocks, were positive for IBV. Regarding spike glycoprotein (S1) gene, sequencing was done to 5 isolates, which showed that they belonged to Eg/12120s/2012 spike glycoprotein (SP1) gene variant 2. Protectotyping was done using 2 isolates, and 3 types of IB live attenuated vaccines (H120, Ma5, IB primer). In conclusion, this study recorded the extensive circulation of variant 2 IBV in Egyptian broiler chickens. Regarding the pathogenicity of the tested variant and cross protection with available live IB vaccines, it was clear that there no complete cross-protection against IBV isolates although of using live attenuated IB vaccines so there a need for further studies to define a more effective program for IB control.
Key words: avian nephritis, infectious bronchitis viruses in broilers in Egypt




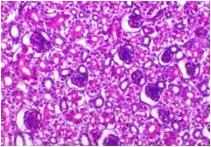
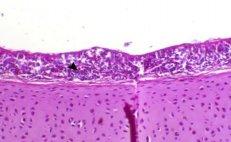
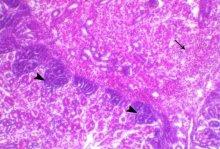
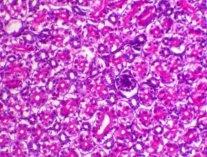

Abdel-Moneim A S, Madbouly H M, Gelb J Jr, and Landman S (2002): Isolation and identification of Egypt/Beni-Suef/01 a novel infectious bronchitis-virus genotype. Vet. Med. J. Giza, 50(4): 1065-1078.
Abdel-Moneim AS, Afifi MA, El-Kady MF (2012) Emergence of a novel genotype of avian infectious bronchitis virus in Egypt. Arch Virol 157: 2453-2457.
Adzhar A, Shaw K, Britton P, and Cavanagh D (1996):Universal digonucleotides for the detection of infectious bronchitits virus by the polymerase chain reaction. Avian Pathol. 25: 817-836.
Adzhar A, Gough R E, Haydon D, Shaw K, Brition P, and Cavanagh D (1997): Molecular analysis of the 793/B Seratype of infectious bronchitis virus in Great Britain. Avian Path, 26: 625-640.
Albassam MA, Winterfield BW, and Thacker HL (1986): Comparison of the nephropathogencity of four strains of infectious bronchitis virus. Avian Dis, 30, 468-476.
Al-Habeeb, A.M; Mohamed, M. H. A. and Sharawi, S. (2013): Detection and characterization of Newcastle disease virus in clinical samples using real time RTPCR and melting curve analysis based on matrix and fusion genes amplification. Vet. World 6(5):239-243.
Ali MMZ (2014): Epidemiological and Molecular studies on the status of Infectious Bronchitis disease in Egypt. Ph.D., Thesis, Poultry Dis.Fac.Vet.Med.Beni-suef Univ.Egypt.
Arafa, A., Samir, M., Hasan, M., Selim, A.,Khaliel, S. (2013): Molecular characterization of infectious bronchitis virus in Egypt, National Laboratory for Veterinary Quality Control on Poultry Production, Animal Health Research Institute, 7 Nadi ElsaidStreet, Dokki, Giza12618, Egypt
Bancroft, J.D. and Stevens, A. (1977): Theory and practices of histologic techniques 2nd Eds. Churchill, Living Stone Edingburgh, London Melborne and New York.
Butcher G D, Winterfield R W, and Shapira D P (1990): Pathogenesis of H13 nephropathogenic infectious bronchitis virus. Avian Dis. 34: 916-921.
Callison S A, M W Jackwood, and D A Hilt, (2001): virus isolates foreign to the United States and associated with nephritis nephrosis syndrome in Molecular characterization of infectious bronchitis chicken. J. Egypt. Vet. Med. Ass., 37(2): 71-79.
Capua I, Minta Z, Karpinska E, Mawditt, Britton P, Cavanagh D, and Gough R E (1999):Co-circulation of four types of infectious bronchitis virus (793/B, 624/I, B1648 and Massachusetts). Avian Pathol. 28: 587-592.
Carstens EB (2010) Ratification vote on taxonomic proposals to the International Committee on Taxonomy of Viruses (2009). Arch Virol 155: 133-146. Cavanagh D (2007) Coronavirus avian infectious bronchitis virus. Vet Res 38: 281-297. 5.
Jackwood MW, Hall D, Handel A (2012) Molecular evolution and emergence of avian gammacoronaviruses. Infect Genet Evol 12: 1305-1311.
Cavanagh D, and Naqi S A(2003): Infectious Bronchitis In: Diseases of Poultry,11thed,Y.M.Saif,H.J.Barsones,J.R.Glisson,A.M.Fa dly,L.R.McDougald and D.E.Swwayne,eds.Iowa State University press ,Amess,Iowa:pp. 101-119.
Cavanagh D, Mawditt K, Britton P, Naylor CJ (1999) Longitudinal field studies of infectious bronchitis virus and avian pneumovirus in broilers using type-specific polymerase chain reactions. Avian Pathology 28: 593– 605.
Cook J.K., Orbell S.J., Woods M.A. and Huggins M.B. (1996): A survey of the presence of a new infectious bronchitis virus designated 4/91 (793B). Vet Rec, 138, 178-180.
Cook, J.K.A., Orbell, S.J., Woods, M.A. & Huggins, M.B. (1999): Breadth of protection of respiratory tract provided by different liveattenuated infectious bronchitis vaccines against challenge with infectious bronchitis viruses of heterologous serotypes. Avian Pathology, 28 , 477-485.
Cowen B S, Widemen R F, Braune M O, and Owen R L (1987): An infectious bronchitis virus isolated from chickens experiencing a urolethiasis outbreak. I. In vitro characterization studies. Avian Dis. 31: 878-883.
De Wit J J, J Nieuwen Huiesen-Van Wilgen, A Hoogkamar, H Van de Sande , G J Zuidam, and T H Fabri (2011):Induction of cystic oviducts and protection against early challenge with infectious bronchitis virus serotype D388(genotype QX)by maternally derived antibodies and by early vaccination .Avian Pathol.40:463-471.
Dhinakar Raj G, Jones RC (1996) Protectotypic di ? ?erentiation of avian infectious bronchitis viruses using an in vitro challenge model. Veterinary Microbiology 53: 239–252.
El-Mahdy, S.S.; El-Hady, M.M. & Soliman,Y. A. (2010): Isolation and characterization of Nephropathogenic strain of infectious Bronchitis virus in Egypt; Journal of American Science 6 (9)669-674.
El-Mahdy SS, Salama E, and Ahmed A (2012): Efficacy of some living classical and variant infectious bronchitis vaccines against local variant isolated from Egypt. Nat Sci; 10(12):292-299.
Even-Chen T, Heifetz S, Perelman B, Kin E, Finger, Avner, Ashash U, And Banet-Noach C (2013): Development of New Infectious Bronchitis Variant 2 Vaccines from a Local Israeli Isolate as a Solution for Regional Virulent Field Strains.World Veterinary Poultry Association French branch (GF-AMVA) 19th - 23rd August 2013.
Frazier JA, Howes K, Reece RL, Kidd AW, Cavanagh D (1990): Isolation of non-cytopathic viruses implicated in the aetiology of nephritis and baby chick nephropathy and serologically related to avian nephritis virus. Avian Pathol. 19: 139-160.
Gelb J Jr, and Jackwood M K (1998):Infectious bronchitis. In: A laboratory manual for the Isolation and Identification of Avian Pathogens. 4th Ed. AAAP: 169- 174.
Gelb Jr J, Weisman Y, Ladman BS, Meir R (2005) S1 gene characteristics and efficacy of vaccination against infectious bronchitis virus field isolates from the United States and Israel (1996 to 2000). Avian Pathol 34: 194- 203.
Ghadakchi H, Pourbakhsh SA, and Hosseini SMH(2005): standardization of an Enzyme –LinkedImmunosorbent Assay for detection of Infectious Bronchitis Virus Antibody ,''Archives of Razi Institute ,59,pp57-83.
Ignjatovic J, and Sapats S (2000): Avian infectious bronchitis virus. Rev. Sci. Off. Int. Epiz. 19: 493-508.
Imada T., Yamaguchi S. and Kawamura H. (1979): Pathogenicity for baby chicks of the G-4260 strain of the picornavirus ‘avian nephritis virus’. Avian Diseases, 23, 582– 588.
Imada T., Yamaguchi, S., Mase M., Tsukamoto K., Kubo, M. and Morooka A (2000): Avian nephritis virus (ANV) as a new member of the family Astroviridae and construction of infectious ANV cDNA. Journal of Virology, 74, 8487_8493.
Jackwood MW, De-wit S (2013) Infectious bronchitis in Diseases of poultry. Swayne DE, Glisson JR, McDougald LR, Nolan LK, Suarez DL et al. John Wiley and Sons Inc Ames Iowa 139–159.
Jackwood MW, Hall D, Handel A (2012) Molecular evolution and emergence of avian gammacoronaviruses. Infect Genet Evol 12: 1305-1311.
Ladman BS, Loupos AB, Gelb JJr (2006) Infectious bronchitis virus S1 gene sequence comparison is a better predictor of challenge of immunity in chickens than serotyping by virus neutralization. Avian Pathology 35: 127–133.
Li M, Mo ML, Huang BC, Fan WS, Wei TC, Li KR, Wei P (2013) Continuous evolution of avian infectious bronchitis virus resulting in different variants cocirculating in southern China. Archives of Virology 158: 1783–1786.
Lim T H, Kim J H, Lee D H, Park J K, Youn H N, Lee J B, Park S Y, Chai I S, and Song C S (2012):live attenuated nephropathogenic infectious bronchitis virus vaccine provide broad protection against new variant strains .Poul Sci.91(1):89-94.
Lohr JE (1988):Differentiation of infectious bronchitis virus strains.In E.F. Kaleta & U. Heffels-Redmann (Eds.), Proceedings of the Ist International Symposium on Infectious Bronchitis Rauischholzhausen, Germany.199-208
Mahgoub K M,Bassiouni A A, Aly M A,and Rabie N S(2010): The prevalence of Infectious Bronchitis (IB)Outbreaks in some chicken farms 3.cross protection of vaccinated chickens versus field IB virus. Journal of American science 6(9):94-108.
Mahmood ZH, Sleman RR, Uthman AU (2011) Isolation and molecular characterization of Sul/01/09 avian infectious bronchitis virus, indicates the emergence of a new genotype in the Middle East. Veterinary Microbiology 150: 21–27.
Malo A, Orbell S, Huggins M, Woods M, and Cook J (1998): Cross protection studies after the use of live attenuated IBV vaccines Nobilis® IB 4-91 and Nobilis® IB Ma5 (Massachusetts type). Intervet, VSD Newsletter: 17:1- 6.
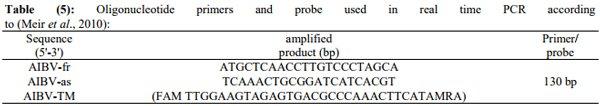
Meir, R.; Maharat, O.; Farnushi, Y. and Simanov, L. (2010): Development of a real-time TaqMan® RT-PCR assay for the detection of infectious bronchitis virus in chickens, and comparison of RT-PCR and virus isolation. J Virol. Methods 163:190–194.
Nguyen TT, Kwonb HJ, Kima H, Hong SM, Seong WJ, Jang JW, Kim JH (2013) Multiplex nested RT-PCR for detecting avian influenza virus, infectious bronchitis virus and Newcastle disease virus. Journal of Virological Methods 188: 41-46.
Nicholas R.A., Goddard R.D., Luff P.R. (1988): Prevalence of avain nephritis virus in England. Veterinary Record, 123, 398.
Parsons D, Ellis M M, Cavanagh D, and Cook J K, (1992): Characterization of an infectious bronchitis virus isolated from IB-vaccinated broiler breeder flocks. Vet. Rec., 131: 408-411.
Purcell DA, Tham VL, and Surman P G (1976): The histopathology of infectious bronchitis in fowls infected with a nephrotropic T strain of virus. Australian Vet. J. 52:85-91.
Reed L J and Muench H (1938):A simple method for estimating fifty percent endpoint. Am. J. Hyg., 27: 493- 496.
Roh HJ, Hilt DA, Williams SM, Jackwooda MW (2013) Evaluation of infectious bronchitis virus Arkansas-type vaccine failure in commercial broilers. Avian Dis 57: 248-259.
Sediek M M (2005): Studies on infectious bronchitis in chickens. M.V.Sc, thesis, poultry Dis, Fac. Vet. Med. Alex Univ. Egypt.
Shirai J, Nakamura K, Shinohara K, Kawamura H (1991) : Pathogenicity and antigenicity of avian nephritis isolates. Avian Dis. 35: 49–54.
Spackman, E; Dennis A. Senne; T. J. Myers; Leslie L. Bulaga; Lindsey P. Garber; Michael L. Perdue; Kenton Lohman; Luke T. Daum and David L. Suarez (2002): Development of a Real-Time Reverse Transcriptase PCR Assay for Type A Influenza Virus and the Avian H5 and H7 Hemagglutinin Subtypes. Journal of Clinical Microbiology, p. 3256–3260
Takase K., Murakawa Y., Ariyoshi R., Eriguchi S., Sugimura, T. and Fujikawa H. (2000): Serological monitoring on layer farms with specific pathogen-free chickens. Journal of Veterinary Medical Science, 62, 1327–1329.
Terregino C, Toffan A, and Serena Beato M (2008): “Pathogenicity of a QX strain of infectious bronchitis virus in specific pathogen free and commercial broiler chickens, and evaluation of protection induced by a vaccination programme based on the Ma5 and 4/91 serotypes,” Avian Pathol. vol. 37, no. 5, pp. 487–493.
Villegas P and Purchase G H (1990): Preparation of chicken embryo kidney cell cultures (CEKC). In: Laboratory manual for the isolation and identification of avian pathogens. AAAP. Ames, Iowa, USA. pp: 3-4.
Winterfield R W and Albassam M A (1984): Nephropathogenicity of infectious bronchitis. Poultry Sc, 63: 2358-2363.
Zhou S, Tang M, Jiang Y, Chen X, Shen X (2014) complete genome sequence of a novel infectious bronchitis virus strain circulating in China with a distinct S gene. Virus Genes 49: 152-15.
















