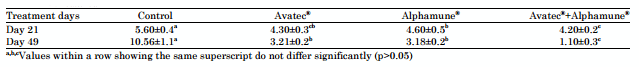INTRODUCTION
Avian coccidiosis is a global parasitic disease characterized with heavy health and economic impacts. Control of this disease is based essentially on chemoprevention using coccidiostats additives and more recently on immunization. Vaccine efficacy is variable and may in some circumstances be restricted, which led to the development of novel protocols that would improve vaccination effectiveness (Allen et al., 2004). Indeed, improving the immunological functions through better hygiene practices and feed additives usage could undoubtedly be a valuable complementary approach (Vermeulen et al., 2001). Several alternative strategies have proven their effectiveness in coccidiosis control with potential stimulatory effects on performances and immunity. They are mainly based on the preservation of the intestinal barrier integrity and the stimulation of the immune response. This is the case of some yeasts and their derivatives (Elmusharaf et al., 2007; Gomez-Verduzco et al., 2009a, b; Oliviera et al., 2009; Swiatkiewicz et al., 2014). Thus, the use of feed additives that prevent diseases such as coccidiosis and promote the nutrients utilization is important.
The present study was carried out to assess the efficiency of some prophylactic protocols based on an ionophore anticoccidial (lasalocid sodium) and a prebiotic (beta-glucans and oligosaccharides) in production performances, preventing coccidiosis and improving broiler immunity under experimental infection conditions.
MATERIALS AND METHODS
Animals and housing: A total of 120 days old ISA 15 chicks were allocated into four equal batches with three replicates in a completely randomized design. Each group was housed in a separate suspended wire cage. Temperature was gradually decreased from 32°C on the first day to 20°C on the 49th day. Continuous lighting was provided with an intensity reduction from the 3rd week. Birds were vaccinated against Newcastle disease and Infectious bursal disease on the first and the 14th day, respectively.
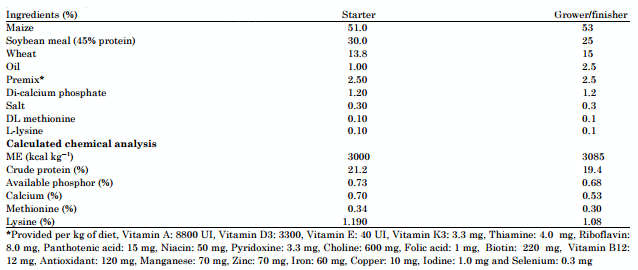
Dietary treatments: To meet the nutrient requirements of the broiler chickens during the experimental period, a complete basal diet was formulated according to AOAC (1990). Table 1 presents the ingredients and the composition of the basal diet. It contains no antibiotic growth promoter, or anticoccidiostatic. Birds of the first batch (A) served as control and received only the basal diet, while those of the other batches (B, C and D) served as tests and they were given the basal diet supplemented respectively with 100 ppm of Avatec® (lasalocid sodium: anticoccidial ionophore) and 0.5 ppm of a prebiotic "Alphamune®" (beta-glucans and oligosaccharides) or a mixture of them, throughout the period of study (J 0-49). Feed and water were provided ad libitum and the trial was performed according to the guidelines established by the Institutional Animal Care and Use Committee.
Avatec® and Alphamune® were provided as premix powders by Alpharma Animal Health (Belgium). During the experimental period, some performance parameters were monitored such as: Live Body Weight (BW) at the 3rd and 7th week of age, daily Feed Intake (FI), feed conversion ratio (FCR) and mortality rate.
Experimental infection with E. acervulina and E. maxima: A native strain of sporulated Eimeria spp. oocysts were obtained from the Department of parasitology (Veterinary and Agricultural Sciences Institute, University of Batna).
Table 1: Composition of the basal diet
Challenge of chickens with E. acervulina and E. maxima was carried out at the 14th day of age. A mixture of E. acervulina (50%) and E. maxima (50%) oocysts were multiplied in broiler chickens. They were conserved in 2% potassium dichloramate solution to induce sporulation and kept refrigerated (3-5°C) until use. To induce the infection, 2 mL of this mix (containing about 1×105 sporulated oocysts) was suspended in saline water and orally administrated to every bird of the trials.
Parasitological and immunological parameters: On the 21st and 49th days, oocysts counts were determined in 10 g of excreta collected along the day. Samples were placed in separate airtight storage bags, mixed thoroughly and kept refrigerated. Then, they were firstly ten-fold diluted in tap water and the resulted solutions were further diluted in saturated NaCl solution at ratio of 1/10 and oocysts were counted using McMaster chambers and presented as the number of oocysts per gram of excreta (Hodgson, 1970).
At these same dates and from each batch, five randomly selected birds were euthanized by jugular vein bleed and the intestinal lesions were noted according to the gross lesion scoring procedure (SLM). A lesion score was assigned from 0-4 (Johnson and Reid, 1970). Blood samples were taken for hematological and biochemical analysis.
Effects of the experimental protocols on the immune system were studied by weighing the lymphoid organs (thymus, bursa of fabricius and spleen) and circulating leucocytes, heterophils and lymphocytes differential counts. The total number of leukocytes counted is 100. Blood samples were taken from the jugular vein for total leukocytes counts.
The lymphoid organs were removed and individually weighed with an analytical balance-A and D-GH-300, then each lymphoid organ index was calculated as the ratio of its weight (g)/body weight (g)×100 (Rizvi and Anjm, 2000). These indices are important criteria in studying immune system status and immune responses during infections or after vaccination (Anwar et al., 2008; Galha et al., 2010).
Total leukocytes counts were estimated using a hemacytometer and Natt and Herrick’s solution. For differential leukocyte counts, the blood smears were prepared and stained with Giemsa stain (Campbell, 1998). Heterophils/Lymphocytes ratio (H/L) was calculated as described by Gross and Siegel (1983) and PCV (packed cell volume) was determined using a microhaematocrit centrifuge (GMDN 32429) at 12000×g for 5 min.
Statistical analysis: Data was subjected to analysis of variance (ANOVA) in the general linear model using the SPSS 17.00 statistical package (SPSS Inc., Chicago, USA). The homogeneity of the variance was tested by Levenes Test. When significant treatment effects will be disclosed at p<0.05, the turkey’s test was applied in order to determine statistical differences between means.
RESULTS
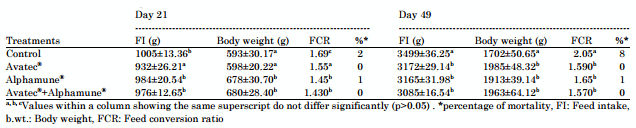
Performance parameters: At the age of 21 days, body weight gain in the control batch was lower than in all the other batches (Table 2) with the highest value in birds of batch D (Avatec®+Alphamune®) followed by group B (Avatec®) and C (Alphamune®).
Feed conversion ratio values in the control group and the Avatec® group were significantly the lowest among all tested groups (p>0.05) and a significant difference (p>0.05) between the groups receiving additives (Avatec® and Alphamune®) and the control group was observed at the end of the trial (49th day). The mortality rate (8%) was the highest in the control group.
Table 2: Performance parameters of broiler chickens
Table 3: Effects of different treatments on lesion scores of infected broiler chickens
Table 4: Effect of dietary treatments on oocysts excretion (log10) per g of excreta in broiler chickens challenged with E. acervulina and E. maxima
Lesions score:
At the end of the starter period (21st day), the average lesions score (SLM) values were lower than one (1.0) for all groups (Table 3). Birds of the control group had recorded the highest SLM value followed by those of group B and C together (0.4) and finally by those of group D, which had the lowest SLM (0.2). There was an increasing tendency of SLM values with age in all groups. At the day 49 the SLM value became 3.0 for group A, indicating a severe infection with E. acervulina and E. maxima. Groups B, C and D had lower SLM values. The highest average lesion score in the 21st and 49th day was found in birds of group A.
There is a significant difference (p>0.05) between the groups that received prophylactic treatment (prebiotic and coccidiostatic) and the control one.
Oocysts excretion: The Table 4 presents the effect of diets on oocyst excretion of broiler chickens challenged with E. acervulina and E. maxima on day14 of age. The number of oocysts per gram of excreta in the control group is higher than the other groups submitted to treatments with a prebiotic or a coccidiostatic or a combination of them (p<0.05).
At the end of the breeding period (day 49), the difference in the number of oocysts excreted in the group that received the association "Avatec®+Alphamune®" is highly significant (p<0.05) compared with Avatec® and Alphamune® groups.
Immunological parameters
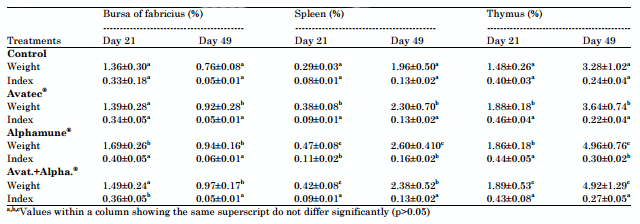
Weight and indices of immune organs: Preventive use of prebiotic Alphamune® and/or anticoccidial Avatec® had beneficial effects on the immunity of birds which resulted in an increase in weight and indices of their lymphoid organs (thymus, bursa and spleen). This increase in weight of the lymphoid organs was significantly (p<0.05) correlated with the increase in body weight of animals of groups Avatec®, Alphamune® and their association (Table 5).
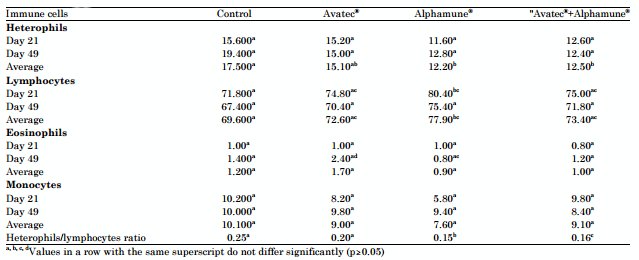
Differential immune cell count: Results showed that there was an improvement in the immunity of birds by stimulating the cellular and humoral immune responses supported by lymphocytes. Birds of group "A" showed a decrease in circulating lymphocytes counts compared to those of other groups. But they recorded the highest heterophils counts and heterophils/lymphocytes ratio. The prebiotic "Alphamune®" boosted the immune response with an increased percentage of circulating lymphocytes compared to the other groups but the difference was not significant (p>0.05) (Table 6).
Table 5: Effects of different treatments on weight and indices of lymphoid organs
Table 6: Effects of the different treatments on the absolute counts of different immune cells. On total, one hundred immune cells were counted
DISCUSSION
The preventive use of anticoccidial Avatec® and/or prebiotic Alphamune® in groups B, C and D improves chickens performances compared to the control group. These results are in agreement with those of De los Santos et al. (2007), Bolu et al. (2009) and Cheraghi et al. (2014), who proved the beneficial effect of prebiotics on growth performances of chickens. According to Gomez-Verduzco et al. (2009a) and despite the beneficial role of yeast derivatives on the body weight, feed intake and feed conversion, these products can be used as feed additives to recover from coccidiosis and to reduce livestock losses due to this disease.
The use of yeast derivatives stimulates broiler performances (body weight and feed conversion), nutrients digestibility as well as the length of intestinal villi (in jejunum, duodenum and ileum) which results in an enlargement of the absorption surface (Zhang et al., 2005; Gao et al., 2008; Al-Mansour et al., 2011). Elwinger et al. (1998) reported that anticoccidial ionophores (monensin and narasin) have an antibacterial activity and they enhance the animal growth in a same way as the antibiotics growth factors avilamicine and avopracin. Mohamed et al. (2008) suggested MOS as alternatives to enramycine® (an antibiotic growth factor) which has more beneficial effects when combined with them.
The low levels of oocysts shedding and the reduced SLM values recorded in our study, demonstrate the success of the different preventive protocols in the control of coccidial replication and confirmed the anticoccidial activity of the used products. However, they do not completely eliminate the parasites from the intestine of birds. Our results are in agreement with those of Elmusharaf et al. (2006) and Gomez-Verduzco et al. (2009a), who have proved the ability of prebiotics and coccidiostat lasalocid sodium to reduce the replication of coccidia.
Lasalocid sodium is an ionophore anticoccidial with excellent activity against all species of Eimeria at a dose of 90 ppm (Botsoglou and Fletouris, 2001). In experimental inoculation, this drug has shown a good anticoccidial activity compared to monensin and salinomycin (Mahmood et al., 2001). In addition, ionophores exhibit an antibacterial activity and reduce the pathogenic enteric bacterial populations such as clostridia that cause infections predisposing to coccidiosis (Chapman et al., 2002).
The protective effect of prebiotics is a result of asexual schizonts development inhibition following the stimulation of local immune mechanisms (Elmusharaf et al., 2006). Elmusharaf et al. (2007) proved the potential anticoccidial ability of MOS to significantly reduce E. acervulina oocysts excretion and intestinal damage. However, these prebiotics still unsuccessful in controlling infestations induced by E. maxima and E. tenella.
The increase in SLM values at the end of our study means that broiler chickens remain susceptible to infestation despite the inclusion of prebiotics and/or anticoccidial lasalocid sodium in their feed as their efficiency can be weakened by many factors. As a solution, Vancraeynest et al. (2009) recommended the increase of ionophore coccidiostats dose to prevent coccidiosis especially with the emergence of drug resistant strains of parasite. A lesional score of 1.8 was observed with a dose of 75 ppm of lasalocid compared to a score of 0.7 with a dose of 125 ppm. Increased litter moisture with age seems to be a predisposing factor to the survival and accumulation of oocysts and facilitate the infestation of poultry (Stanley et al., 2004). It is worth noting that the immune response developed following a primary infestation may not be sufficient to protect the chicken at advanced ages. Christophe (2007) chickens of more than 28 days old still sensitive to coccidian infections and severe intestinal lesions could be observed even beyond 42-45 days of age. The incompletion of the immunity against chicken coccidia seems to be a plausible explanation.
Several hypotheses attempted to explain the beneficial effect of MOS products in avian coccidiosis control. Indeed, these products have the ability to simultaneously stimulate cellular immune response (which plays a major role in controlling intestinal parasitism) and local production of secretory antibodies during natural exposure to Eimeria spp. (Gomez-Verduzco et al., 2009a).
Nollet et al. (2007) reported that the association of MOS with vaccination increases the resistance of chickens to coccidia infestation with stimulation of their immune response and production performances. The inclusion of prebiotics enhances the cellular (lymphocytes proportion) and humoral (IgA titer in the ileum) immune responses (Li et al., 2007; Swanson et al., 2002). Furthermore, they protect the intestinal mucosa in the case of inflammatory reactions induced by pathogens or toxins by increasing the villi length and facilitating their regeneration (Gao et al., 2008). The MOS can compete with sporozoites for binding sites on intestinal epithelial cells and thereby reduce the adhesion and the subsequent proliferation of parasites (Shoaf et al., 2006).
The bursa of fabricius, spleen, thymus and lymphoid tissue associated to the intestine (cecal tonsils) are major actors in the immune response against intestinal pathogens (Lillehoj and Lillehoj, 2000). Parasitic infestation stress causes a decrease in body weight gain and feed consumption and affects the immune system of birds (Rizvi and Anjum, 2000). Reduced lymphoid organs indices are typical in birds suffering from mixed coccidial infections (E. tenella, E. acrervulina, E. maxima and E. necatrix) (Anwar et al., 2008). These affected organs show micro-necrosis and lack of immune cells migration (Olariu-Jurca et al., 1994). Many defense mechanisms are involved in the protection from coccidian infection. The cellular immune response supported by the T lymphocytes enhances resistance to coccidiosis (Pakandl et al., 2008) and cytokines intensify the level of this protection. The increase in Interferon gamma levels (IFN-γ, transcripted in the spleen and cecal tonsils) is particularly associated with protective immunity against coccidiosis (Yun et al., 2000; Lillehoj et al., 2004). Antibodies (IgA, IgG and IgM) production begins soon after natural infection (Lillehoj and Lillehoj, 2000) or vaccination (with E. tenella antigen) especially in the spleen (Ayaz et al., 2008) with an effectual protection of intestinal mucosa and a significant diminution in clinical signs severity and mortality rates.
The stimulation of the performances of birds with anticoccidials and/or prebiotics can influence the immune response due to their action on weight and immune organs indices. Yalcinkaya et al. (2008) confirmed the impact of MOS on weight of the internal organs (liver and pancreas) as well as organs of the immune system. A dose of 0.05% MOS significantly increases the weight of the bursa of fabricius. In chickens receiving anticoccidials or prebiotics in their diets, body weight improvement comes along with an increase in weight of internal and lymphoid organs (Stanley et al., 2004; Kadam et al., 2009).
Increased Heterophis/Lymphocytes ratio is a major indicator of a stress condition (Gross and Siegel, 1983). According to Duffy et al. (2005), intestinal parasitism is an important stress factor in poultry and the inflammatory reaction that accompanies intestinal lesions involves the heterophils participation (Stanley et al., 2004).
Lasalocid sodium and prebiotic supplementation also results in increased percentage of circulating lymphocytes and decreased H/L ratio, this leads to enhancing cellular immune response and increasing antibodies production. According to Bolu et al. (2009) and Gomez-Verduzco et al. (2009a) the prebiotic "Alphamune®" enhances cellular immune response with a significant increase of leukocytes especially lymphocytes. The immuno-stimulating effect of prebiotics is related to an amplification of the humoral (antibody titer) and cellular (percentage of lymphocytes) immune responses (Li et al., 2007; Rugea et al., 2009). In addition to this stimulation of the immunity at the peripheral level, prebiotics also locally increase the immunoglobulin titer (IgA) in the ileum (Swanson et al., 2002). Cellular immune response can also be enhanced by ionophores addition (Munir et al., 2009). Prebiotics and ionophores used to prevent coccidiosis can indirectly stimulate the immunity in post vaccination of poultry against certain immunosuppressive diseases affecting the lymphoid organs or against infections predisposing to coccidiosis such as Marek's disease which affects the T cell response (Morimura et al., 1995). Thus, the MOS enhances the immune response by increasing the antibody titer during vaccination against infectious bursal disease (Oliviera et al., 2009) and Newcastle disease (Gomez-Verduzco et al., 2009a; Oliviera et al., 2009).
In contrast, Rizvi and Anjum (2000) reported the absence of a significant effect of various doses of salinomycin (an anticoccidial ionophore) on indices of lymphoid organs. A very high dose of this drug (180 ppm) may cause immuno-suppression in poultry with a reduction in total leucocytes and lymphocytes count. However, this effect seems to be dose dependent. A dose of 90 ppm of this anticoccidial stimulates the humoral immune response in post vaccination and doses greater than 90 ppm may even give more satisfactory results (Jafari et al., 2010).
CONCLUSION
The prophylactic use of anticoccidial ionophore lasalocid sodium (Avatec®,100 ppm) and/or prebiotic (Alphamune®,0,5 ppm) as feed additives throughout the rearing period, improves the health of broiler chickens by stimulating their growth performances (weight gain, feed intake and feed conversion ratio) and immune responses (weight and indices of immune organs lymphocytes count). It results also in a better control of coccidiosis (decreased oocyst excretion and severity of intestinal lesions).
The association of Avatec® and Alphamune® is the best treatment, which demonstrate interesting synergistic effects and seem to be promising alternatives in the control of coccidian infections, because of the emergence of drug-resistance among Eimeria strains in addition to the cost of vaccination.
ACKNOWLEDGMENTS
The authors wish to express their gratitude to the Biolab (Algeria) and Alpharma Animal Health (Belgium) Companies, for supporting the present research by kindly donating the coccidiostatic and prebiotic.
REFERENCES
AOAC., 1990. Official Methods of Analysis of the Association of Official Analytical Chemists. 13th Edn., Association of Official Analytical Chemist, Washington, DC., USA.
Al-Mansour, S., A. Al-Khalf, I. Al-Homidan and M.M. Fathi, 2011. Feed efficiency and blood hematology of broiler chicks given a diet supplemented with yeast culture. Int. J. Poult. Sci., 10: 603-607.
Allen, P.C., M. Anderson and H.D. Danforth, 2004. Use of echinacea as a feed additive to enhance protection against coccidiosis. United States Department of Agriculture Patents No. US 6,767,546 B1, pp: 1-10. http://naldc.nal.usda.gov/catalog/6452.
Anwar, M.I., M. Akhtar, I. Hussain, F. Muhammad and A.U. Haq, 2008. Effects of local gametocyte and livacox vaccines on live body weight gain and lymphoid organs in chickens. Pak. Vet. J., 28: 136-138.
Ayaz, M.M., M. Akhtar, I. Hussain, F. Muhammad and A.U. Haq, 2008. Immunoglobulin producing cells in chickens immunized with Eimeria tenella gametocyte antigen vaccines. Veterinarni Medicina, 53: 207-213.
Bolu, S.A., V. Ojo, O. Oluyemi, O.I. Babawale and O.A. Awodele, 2009. Effect of graded level of alphamune g on performance, blood chemistry and histology of cockerel chicks. Int. J. Poult. Sci., 8: 397-400.
Botsoglou, N.A. and D.J. Fletouris, 2000. Drug Residues in Foods Pharmacology: Food Safety and Analysis. 1st Edn., CRC Press, USA., ISBN-13: 978-0824789596, Pages: 1200.
Campbell, T.W., 1998. Avian Hematology and Cytology. Iowa State University Press, Ames, USA., pp: 104.
Chapman, H.D., T.E. Cherry, H.D. Danforth, G. Richards, M.W. Shirley and R.B. Williams, 2002. Sustainable coccidiosis control in poultry production: The role of live vaccines. Int. J. Parasitol., 32: 617-629.
Cheraghi, A., H. Khosravinia, S.M. Mousavi and B. Massori, 2014. Effects of dietary levels of yeast extracted β-glucans and α-mannans (AlphamuneTM) on performance of broiler chicken raised in normal and thermal-stressed conditions. Eur. Poult. Sci., 78: 63-68.
Christophe, B., 2007. Master of coccidiosis in broilers-a national record HTS database and applications of new data in the mastery of coccidiosis production organizations. Seventh Poultry Research Days, pp: 348-351.
De los Santos, F.S., A.M. Donoghue, M.B. Farnell, G.R. Farnell, G.R. Huff, W.E. Huff and D.J. Donoghue, 2007. Gastrointestinal maturation is accelerated in turkey poults supplemented with a mannan-oligosaccharide yeast extract (Alphamune). Poult. Sci., 86: 921-930.
Duffy, C.F., G.F. Mathis and R.F. Power, 2005. Effects of Natustat™ supplementation on performance, feed efficiency and intestinal lesion scores in broiler chickens challenged with Eimeria acervulina, Eimeria maxima and Eimeria tenella. Vet. Parasitol., 130: 185-190.
Elmusharaf, M.A., V. Bautista, L. Nollet and A.C. Beynen, 2006. Effect of a mannanoligosaccharide preparation on Eimeria tenella infection in broiler chickens. Int. J. Poult. Sci., 5: 583-588.
Elmusharaf, M.A., H.W. Peek, L. Nollet and A.C. Beynen, 2007. The effect of an in-feed Mannanoligosaccharide preparation (MOS) on a coccidiosis infection in broilers. Anim. Feed Sci. Technol., 134: 347-354.
Elwinger, K., E. Berndtson, B. Engstrom, O. Fossum and L. Waldenstedt, 1998. Effect of antibiotic growth promoters and anticoccidials on growth of Clostridium perfringens in the caeca and on performance of broiler chickens. Acta Veterinaria Scandinavica, 39: 433-441.
Galha, V., E.F. Bondan, L.V. Bonamin and M.A. Lallo, 2010. [Clinical coccidiosis in broiler chicks naturally infected and immunosuppressed with dexamethasone]. Arq. Inst. Biol., 77: 25-31, (In Portuguese).
Gao, J., H.J. Zhang, S.H. Yu, S.G. Wu and I. Yoon et al., 2008. Effects of yeast culture in broiler diets on performance and immunomodulatory functions. Poult. Sci., 87: 1377-1384.
Gomez-Verduzco, G., A. Cortes-Cuevas, C. Lopez-Coello, E.M. Avila-Gonzalez and G. Nava, 2009a. Dietary supplementation of mannan-oligosaccharide enhances neonatal immune responses in chickens during natural exposure to Eimeria spp. Acta Veterinaria Scandinavica, Vol. 51.
Gomez-Verduzco, G., A. Cortes-Cuevas, C. Lopez-Coello, J. Arce-Menocal, C. Vasquez-Pelaez and E. Avila-Gonzalez, 2009b. Productive performance and immune response in broilers fed a sorghum+soy diet supplemented with yeast (Saccharomyces cerevisiae) cell walls, in the presence or absence of aflatoxin B1. Tec. Pecu. Mex., 47: 285-297.
Gross, W.B. and H.S. Siegel, 1983. Evaluation of the heterophil/lymphocyte ratio as a measure of stress in chickens. Avian Dis., 27: 972-979.
Hodgson, J.N., 1970. Coccidiosis: Oocyst-counting technique for coccidiostat evaluation. Exp. Parasitol., 28: 99-102.
Jafari, R.A., M. Ghorbanpoor and M.A. Behmanesh, 2010. Effects of Salinomycin on growth performance and immune response of broiler chicks vaccinated against infectious bursal disease virus. Proceedings of the 2nd International Veterinary Poultry Congress, February 20-21, 2010, Tehran, Iran.
Johnson, J. and W.M. Reid, 1970. Anticoccidial drugs: Lesion scoring techniques in battery and floor-pen experiments with chickens. Exp. Parasitol., 28: 30-36.
Kadam, A.S., V.D. Lonkar, V.R. Patodkar, S.M. Kolangath and T.A. Bhosale, 2009. Comparative efficacy of supplementation of natural (Citrous limon Juice), herbal and synthetic vitamin c on the immune response of broiler chicken during summer stress. Asian J. Poult. Sci., 3: 57-62.
Li, G.H., B. Liu, M.R. Qu, X.F. Zhang, G.B. Liu, M. Gao and A.Z. Zhang, 2007. Effects of soybean oligosaccharides infusion in different parts of the gastrointestinal tract on several immune indices in sheep. Chin. J. Anim. Nutr., 19: 1-7.
Lillehoj, H.S. and E.P. Lillehoj, 2000. Avian coccidiosis. A review of acquired intestinal immunity and vaccination strategies. Avian Dis., 44: 408-425.
Lillehoj, H.S., W. Min and R.A. Dalloul, 2004. Recent progress on the cytokine regulation of intestinal immune responses to Eimeria. Poult. Sci., 83: 611-623.
Mahmood, A., M.A. Khan, M.N. Khan and A. Qudoos, 2001. Effect of ionophores on some parameters of broilers experimentally infected with Eimeria species. Int. J. Agric. Biol., 3: 469-471.
Mohamed, M.A., H.M.A. Hassan and E.M.A. El-Barkouky, 2008. Effect of mannan oligosaccharide on performance and carcass characteristics of broiler chicks. J. Agric. Soc. Sci., 4:: 13-17.
Morimura, T., M. Hattori, K. Ohashi, C. Sugimoto and M. Onuma, 1995. Immunomodulation of peripheral T cells in chickens infected with Marek's disease virus: Involvement in immunosuppression. J. Gen. Virol., 76: 2979-2985.
Munir, K., M.A. Muneer, A. Tiwari, E. Masaoud and R.M. Chaudhry, 2009. Effects of salinomycin on cell-mediated immunity of broiler chickens against hydropericardium syndrome and Newcastle disease viruses. Poult. Sci., 88: 86-91.
Nollet, L., G. Huyghebaert and P. Springs, 2007. Effect of dietary mannan oligosaccharide on live performance of broiler chickens given an anticoccidial vaccination followed by a mild coccidial challenge. Proceedings of the 16th European Symposium on Poultry Nutrition, August 26-30, 2007, Strasbourg, France, pp: 365-368.
Olariu-Jurca, I., M. Coman, G. Manu and C. Falea, 1994. Microscopic, histological and haematological studies of chicken with coccidiosis. Lucrari-Scientific, 28: 53-56.
Oliviera, M.C., D.F. Figueiredo-Lima, D.E.F. Filho, R.H. Marques and V.M.B. Moraes, 2009. Effect of mannanoligosaccharides and/or enzymes on antibody titers against infectious bursal and newcastle disease viruses. Arquivo Brasileiro Medicina Veterinaria Zootecnia, 61: 6-11.
Pakandl, M., L. Hlaskova, M. Poplstein, M. Neveceralova, T. Vodicka, J. Salat and J. Mucksova, 2008. Immune response to rabbit coccidiosis: A comparison between infections with Eimeria flavescens and E. intestinalis. Folia Parasitologica, 55: 1-6.
Rizvi, F. and A.D. Anjum, 2000. Effect of salinomycin on immunity of broiler chicks. Pak. J. Biol. Sci., 3: 1940-1942.
Rugea, T., R. Trif, E. Tirziu, I. Nichita, C. Cumpanasoiu, R.V. Gros and M. Seres, 2009. Immunomodulator and nutritional effects of bio-mos. Lucrari Stiintifice Zootehnie si Biotehnologi., 42: 399-403.
Shoaf, K., G.L. Mulvey, G.D. Armstrong and R.W. Hutkins, 2006. Prebiotic galactooligosaccharides reduce adherence of enteropathogenic Escherichia coli to tissue culture cells. Infect. Immun., 74: 6920-6928.
Stanley, V.G., C. Gray, M. Daley, W.F. Krueger and A.E. Sefton, 2004. An alternative to antibiotic-based drugs in feed for enhancing performance of broilers grown on Eimeria spp.- infected litter. Poult. Sci., 83: 39-44.
Swanson, K.S., C.M. Grieshop, E.A. Flickinger, L.L. Bauer and H.P. Healy et al., 2002. Supplemental fructooligosaccharides and mannanoligosaccharides influence immune function, ileal and total tract nutrient digestibilities, microbial populations and concentrations of protein catabolites in the large bowel of dogs. J. Nutr., 132: 980-989.
Swiatkiewicz, S., A. Arczewska-Wlosek and D. Jozefiak, 2014. Immunomodulatory efficacy of yeast cell products in poultry: A current review. World's Poult. Sci. J., 70: 57-68.
Vancraeynest, D., M. de Gussem, F. Nerat, M. Marien, G. Fort and M. Naciri, 2009. Interest of increased coccidiostats doses for the prevention of coccidiosis. Eighths Poultry Research Days, St Malo, March 25-26, 2009, pp: 1-5.
Vermeulen, A.N., D.C. Schaap and T.P.M. Schetters, 2001. Control of coccidiosis in chickens by vaccination. Vet. Parasitol., 100: 13-20.
Yalcinkaya, I., T. Gungor and M. Basalan, 2008. Effects of mannanoligosaccharide (MOS) from saccharomyces cerevisiae on some internal, gastrointestinal and carcass parameters in broilers. J. Anim. Vet. Adv., 7: 789-792.
Yun, C.H., H.S. Lillehoj and E.P. Lillehoj, 2000. Intestinal immune responses to coccidiosis. Dev. Comp. Immunol., 24: 303-324.
Zhang, A.W., B.D. Lee, S.K. Lee, K.W. Lee, G.H. An, K.B. Song and C.H. Lee, 2005. Effects of yeast (Saccharomyces cervisiae) cell components on growth performance, meat quality and ileal mucosa development of broiler chicks. J. Poult. Sci., 84: 1015-1021.
Asian Journal of Poultry Science 9 (3): 133-143, 2015 ISSN 1819-3609 / DOI: 10.3923/ajpsaj.2015.133.143 © 2015 Academic Journals Inc.