Introduction
Fruits and vegetables, whole grains, beverages, and some fortified or omega-3 enriched products can be considered as functional foods. In fact, consumption of omega-3 is associated with reducing the risk of cardiovascular diseases, and cancers (Mozaffarian et al., 2005; Theodoratou et al., 2007) besides improving animal health and blood parameters (Jameel and Sahib, 2014). Tissue structure, muscles are the target or location of the fatty acid deposition. Modifying the dietary fatty acid profile stimulates α-linolenic acid enrichment in the triacylglycerol fraction of the meat (De La Ossa, 2009). Meat industry develops to produce healthier meat characterize by reduction of fat content, modification of fatty acid composition, incorporation of functional ingredients, reduction of calories, nitrites and cholesterol content (Jimenez-Colmenero et al., 2001). However, high levels of unsaturated fatty acids in broiler diet lead to increasing the degree of unsaturation muscle membrane lipids that cause, reducing oxidative stability of the meat (Morrissey and Kiely, 2006). These mechanisms result in the formation of oxysterol especially when meat is exposed to light, radiation, long-term storage and elevated temperatures during cooking. Hens fed high levels of antioxidants produced eggs with higher concentrations of antioxidants and chicks hatched from those eggs had higher concentrations of antioxidants in their tissues (Cherian et al., 1997; Surai et al., 1999). Supplementing the hen’s diet with oils containing beneficial omega-3 fatty acids and high levels of antioxidants can be costly. The ability to direct supply growing embryos with specific compounds may decrease the need for the long term formulation of enriched rations for maternal diets to achieve a similar effect, therefore, In ovo injections may also provide a more accurate dose at a specific time for peak absorption of specific nutrients by the embryo (Schaal, 2008).As such, In ovo administration of high quality fatty acids may prove beneficial for improving energy production during embryogenesis and hatching (Al-Zuhairy and Alasadi, 2013). The use of antioxidants, especially vitamin E has been proven to reduce harmful peroxidation of lipids and cholesterol in animal models (Singh et al., 2005). Developed and improved nutritional status afforded by In ovo feeding subsequently improved hatchability percentage, hatching weight, and growth performance and immune response besides increasing body weight at market age (Selim et al., 2012; Al-Zuhairy and Alasadi, 2013). This study was designed to investigate the effects of In ovo injection with Newcastle disease vaccine, multivitamins AD3E, and omega-3 on carcass characteristics of broilers.
Materials and Methods
Experimental design
An experiment was conducted on 400 fertilized commercial broiler eggs (Cobb strain). All eggs were distributed into four treatments, 100 eggs per each treatment. The incubation period was carried out at Al-Saud Hatchery in the holy city of Karbala. All fertilized eggs for each experiment were set in the trays in the same incubator. On day 18 of incubation, all the eggs were transferred from incubators to hatchers, (Sharma and Burmester, 1982, showed that day 18 of incubation was the best time of In ovo injection) to facilitate the In ovo injections. The eggs were first candled and clear eggs (non-fertile) were removed. Eggs were cleaned with 70% ethanol and small puncture was made using a modified machine that approach all eggshells. Eggs were injected through 23 gauge, 1.25 inch needle and automatic injector to administer all injections into the amnion of the egg. The injection of In ovo was done as follow: 100 fertilized eggs injected In ovo with 0.1ml saline solution as control group (T1), 100 fertilized eggs injected In ovo with 0.1ml killed Newcastle disease vaccine (T2), 100 fertilized eggs injected In ovo with 0.1ml killed Newcastle disease vaccine and 0.1 ml multivitamins AD3E (T3), and 100 fertilized eggs injected In ovo with 0.1ml killed Newcastle disease vaccine and 0.1 ml omega-3 (T4). Upon completion of all injections, all eggs were returned to the hatcher until the day of hatching. The hatched chicks were boxed and transported from hatchery to poultry farm of College of Veterinary Medicine / University of Baghdad and distributed into treatments and each treatment group was further sub-divided into 2 replicates.
Feeding program
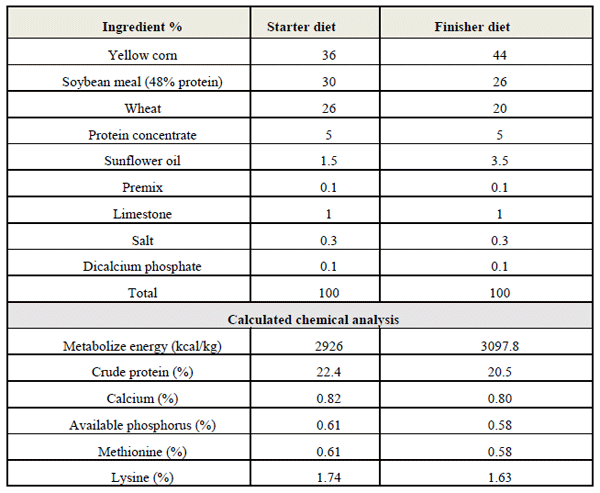
Feed and water provided in “ad libitum” during the experiment. A two-phase of feeding program, consists of offering a starter (1-21 days of age) and finisher (22-35 days of age) was provided to the broilers (Table 1).
Table (1) compositions of experimental diets (NRC, 1994)
Carcass characteristics
At day 35th of age, six birds were taken from each replicate randomly and weighted individually using digital balance and then slaughtered. The head, feather, viscera, and legs were removed and washed. Each carcass was weighted to get the dressing percentage according to the routine methods as described by Al-Fayadh and Naji (1989). The edible giblets (heart, liver and gizzard) were separated from other organs and tissues then weighted using an electrical balance, relative weight as percentage was calculated. Thigh and breast meat without skin and bone was separated from the carcass and placed in plastic container and stored in deep freeze (-20ºC) until analysis. Lipid of meat was extracted by using diagnostic cholesterol kit (Allain et al., 1974).
Statistical analysis
All Data were analysed statistically by using analysis of variance (ANOVA) and means compared for significance using least significant difference (L.S.D) for comparison of means on a computer program by using SPSS program (Snedecor and Coehran, 1980).
Results and Discussion
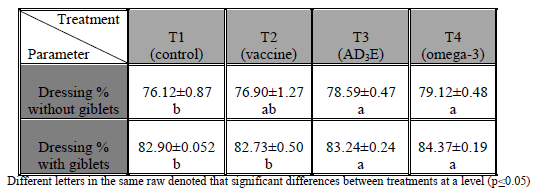
The effects of In ovo injection with ND vaccine, multivitamin AD3E, and omega-3 on dressing percentage without giblets and with giblets were presented in (Table 2) and improved significantly (p≤0.05) in T4 (In ovo injected with ND vaccine and omega-3) and T3 (In ovo injected with ND vaccine and multivitamins AD3E) respectively as compared with T2 (In ovo injected with ND) and with control group.
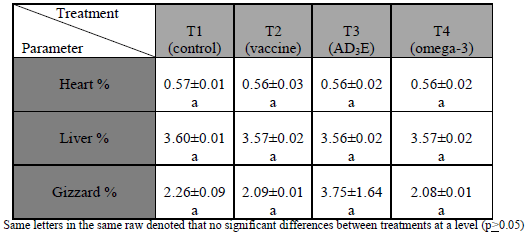
Relative weights of edible giblets (heart, liver and gizzard) were presented in (Table 3) and there were no significant differences (p≤0.05) among treated groups.
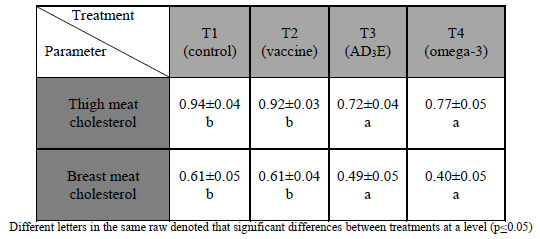
Thigh meat cholesterol and breast meat cholesterol were presented in (Table 4) and decreased significantly (p≤0.05) in T4 (In ovo injected with ND vaccine and omega-3) and T3 (In ovo injected with ND vaccine and multivitamins AD3E) respectively as compared with T2 (In ovo injected with ND and with control group.
The reduction of cholesterol in thigh meat and breast meat could be due to omega-3 PUFAs reduces triglycerides by decreasing hepatic synthesis and the secretion of triglyceride-rich lipoproteins (very low density lipoproteins) by inhibiting various enzymes due to their effects on the expression of specific gene than inhibiting various enzymes lead to decrease hepatic synthesis and secretion of VLDL.
Omega-3 suppressing lipoprotein lipase activity, an enzyme that hydrolyzes triglyceride from VLDL particles when reached the tissue (Lehninger, 1982). Omega-3 may reduce serum cholesterol due to these oils inhibition liver enzymes 5-hydroxy-3-methylglutaryl-coenzyme a reductase (HMG-Co A) (Lehninger, 1982). Flaxseed oil may control on the expression of specific genes that may lead to raise the ability of liver for HDL synthesis (Sessler and Ntambi, 1998). Also omega-3 increases the excretion of bile, chemical nature of chicken bile characterized by containing high proportion of cholesterol and triglycerides instead of phospholipids as in mammals (Griminger, 1985) that makes the bile act as a container for lipid drain in chicken; Therefore any effect which prevents the reabsorption of it in intestine or increases its output considers as potency effect in lowering blood cholesterol specifically and the lipid generally (Weiss and Scott, 1979).
Table (2) Effect of in ovo injection on dressing percentage (%). Mean ± standard error
Table (3) Effect of in ovo injection on relative weights of edible giblets (%). Mean ± standard error
Table (4) Effect of in ovo injection on meat cholesterol (mg/gm).Mean ± standard error
The results of the experiment are in agreement with suggestion (Dolnikoff et al.; 2001) they were recorded that the use of vitamin E and omega-3 have been reduced triglycerides and cholesterol concentration then lead to decrease serum LDL and VLDL concentration. Also El-Yamany et al. (2008) showed that significantly reduction of cholesterol and triglycerides in serum and meat after feeding quail with flaxseed oil or fish oil.
The best strategy to improve the ratio is by increasing the levels of omega-3 PUFAs in the meat. An effective approach for raising omega-3 PUFAs consumption is by increasing omega-3 PUFAs levels in edible muscle tissues (Rymer and Givens, 2005). However, omega-3 enriched chicken meat contains more double bonds, which leads to a higher lipid oxidation rate (Barroeta, 2007). In this regard, the meat industry is looking forward to develop new strategies in terms of antioxidant supply required to limit lipid oxidation, which adversely affects meat nutritional value and sensory attributes. Polyunsaturated fatty acids in meat which oxidizes through a free-radical chain mechanism (Li et al., 1996). (Engberg et al., 1996) found that α-tocopherol retention in broilers was significantly reduced when oxidized oil was incorporated in the diet. As a consequence, using oxidized oils in the diet requires a higher level of dietary vitamin E to maintain the antioxidant/pro-oxidant balance in muscle membranes (Mahoney and Graf, 1986). Dietary vitamin E supplementation period definitely influences the oxidative stability in meat (Morrisey et al., 1998). The use of antioxidants, especially vitamin E has been proven to reduce harmful peroxidation of lipids and cholesterol in animal models (Singh et al., 2005).
Dressing percentage without and with giblets were increased while relative weights of edible giblets had no effect may be related to approximately during the final week of age, the birds directed to muscle building in addition could be due to the important role of exogenous omega-3 and vitamin E in reducing the production of free radicals that cause a serious damage to the cellular membranes (Cherian and Sim, 1992, 1997; Cherian et al., 1997), also exogenous omega-3 and vitamin E decreasing serum VLDL may decrease fat deposition in the body. Finally increasing dressing percentage above mean providing more meat for consumers. The current results agree with (´pez-Ferrer et al., 2001; Lember, 2006; Chashnidel et al., 2010; Mansoub, 2011; Sahito et al., 2012).
Conclusion
In conclusion, this study approved that In ovo injection of ND vaccine with omega-3 or with multivitamins AD3E could improve dressing percentage, thigh meat cholesterol and breast meat cholesterol of broilers.
References
Al-Fayadh H A and Naji S. (1989). Technologyia poultry production.1st ed. College of Agriculture. University of Baghdad Press. Iraq. (In Arabic).
Allain CC, Poon L S, Chan CS, Richmond W and Fu PC. (1974). Enzymatic determination of total cholesterol. Clin. Chem. 20, (4):470-475.
Al-zuhairy MA and Alasadi Y j. (2013). Effect of in ovo injection with Newcastle disease vaccine, multivitamins AD3E, and omega-3 on performance and immune response of broiler. I. J. A. B. R. 3,(2): 208-211.
Barroeta, A. C. (2007). Nutritive value of poultry meat: Relationship between vitamin E. and PUFA .World's. J. Poult. Sci. 63(2): 277-284.
Chashnidel Y, Moravej H, Towhidi A, Asadi F and Zeinodini S. (2010). Influence of different levels of n-3 supplemented (fish oil) diet on performance, carcass quality and fat status in broilers. Afr. J. Biotechnol. 9, (5): 687-691.
Cherian G and Sim J. (1992). Preferential accumulation of n-3 fatty acids in the brain of chicks from eggs enriched with n-3 fatty acids. Poult. Sci.71: 1658-1668.
Cherian G and Sim J S. (1997). Egg yolk polyunsaturated fatty acids and vitamin E content alters the tocopherol status of hatched chicks. Poult. Sci. 76: 1753-1759.
Cherian G, Gopalakrishnan N, Akiba Y and Sim JS. (1997). Effects of maternal dietary 18:3 n-3 acids on the accretion of long chain polyunsaturated fatty acids in the tissue of developing chick embryo. Biol. Neonate. 72:165-174.
DeLa Ossa TIP. (2009). Omega-3 enrichment and oxidative stability of broiler chicken meat.Msc. Thesis in food science and technology. Alberta University.
Dolnikoff M, Martin-Hidalgo A, Machado UF, Lima F B and Herrera E. (2001). Decreased lipolysis and enhanced glycerol and glucose utilization by adipose tissue prior to development of obesity in monosodium glutamate (MSG) treated-rats. Int. J. Obes. Relat. Metab. Disord. 25: 426-433.
El-Yamany AT, El-Allawy HMH, El-Samee LDA and EL-Ghamry AA. (2008). Evaluation of using different levels and sources of oil in growing Japanese quail diets. American-Eurasian. J. Agric. and Environ. Sci. 3,(4): 577-582.
Engberg RM, Lauridsen CS, Jensen K and Jacobsen K. (1996). Inclusion of oxidized vegetable oil in broilers diet. Its influence on nutrient balance and on the antioxidative status of broilers. J. Poult. Sci. 75: 1003-1011.
Griminger P. (1985). Lipid metabolism. In: Avian physiology. (Sturkie, P.D). 4th ed. Springer-Verlag New York. Berlin heidelberg. Tokyo. 345-358.
Jameel YJ and Sahib AM. (2014). Study of some Blood Parameters of Broilers Fed on Ration Containing Fish Oil. Journal of Biology, Agriculture and Healthcare. ISSN 2224-3208 (Paper) ISSN 2225-093X (Online). 4, (7): 67-71.
Jimenez-Colmenero F, Carballo J and Cofrades S. (2001). Healthier meat and meat products: their role as functional foods. Meat. Sci. 59: 5-13.
Lehninger A L (1982). Principles of biochemistry. Worth Publishers Inc. 607.
Lember A, Tikk H Tikk V, Tamm K, Karus A, Kuusik S and Rei m. (2006).The use of linseed oil in enriching the lipids of hen broiler, quail and rabbit meat with ω-3 fatty acids. Journal of agricultural science.17 (1): 45-67.
Li SX, Ahn DU, Cherian G, Chung TY and Sim JS. (1996). Dietary oils and tocopherol supplementation on cholesterol oxide formation in freeze-dried chicken meat during storage. J. Food Lipids. 3: 27-42.
Lo´pez-Ferrer S, Baucells MD, Barroeta AC and Grashorn MA. (2001).n-3 enrichment of chicken meat use of very long-chain fatty acids in chicken diets and their influence on meat quality: fish oil. Int. J. Poult. Sci., 80: 741-752.
Mahoney JR and Graf E. (1986). Role of alpha-tocopherol, ascorbic acid, citric acid and EDTA as oxidants in model systems. J. Food Sci. 51: 1293-1296.
Mansoub NH. (2011). Effect of fish oil fed a low-protein diet on performance, carcass characterizes and blood indices in broiler chicks. Annals of biological research. 2(3): 113-120.
Morrisey PA, Sheehy PJ A, Galvin K, Kerry JP and Buckle DJ. (1998). Lipid stability in meat and meat products. Meat Sci. 49: S73-S86.
Morrissey PA and Kiely M. (2006). Oxysterols: formation and biological function. In: Advanced dairy chemistry Vol. 2 lipids (Fox, P. F. and McSweeney, P. L. H.). 3rded. Springer. New York.
Mozaffarian D, Ascherio A, Hu FB, Stampfer MJ, Willett WC, Siscovick DS and Rimm EB. (2005). Interplay between different polyunsaturatedfatty acids and risk of coronary heart disease in men. Circulation 111:157-164.
National Research Council (NRC). (1994). Nutrient requirements of poultry.9th ed. National Academy Press. Washington. D. C. USA.
Rymer C and Givens D I. (2005).n-3 fatty acid enrichment of edible tissue of poultry: A review. Lipids, 40(2): 121-130.
Safamerhr A, Aghaei N and Mehmannavaz Y. (2008).The influence of different levels of dietary fish oil on the performance, carcass traits and blood parameters of broiler chickens. Res. J. Biol. Sci. 3(10): 1202-1207.
Sahito HA, Soomro RN, Memon A, Abro MR,Ujjan NA and Rahman A. (2012). Effect of fat supplementation on the growth, body temperature and blood cholesterol level of broiler.Glo. Adv. Res. J. Chem. Mat. Sci. 1,(2): 023-034.
Schaal TP. (2008). The effects of in ovo feeding of fatty acids and antioxidants on broiler chicken hatchability and chick tissue lipids. University Honors College. Oregon State University.
Selim SA, Gaafar KM and El-ballal SS. (2012).Influence of in-ovo administration with vitamin E and ascorbic acid on the performance of Muscovy ducks. Emir. J. Food. Agric. 24(3): 264-271.
Sessler A M and Ntambi JM. (1998).Polyunsaturated fatty acid regulation of gene expression. The Journal of nutrition. 128,(6): 923-926.
Sharma JM and Burmester BR. (1982). Resistance to Marek’s disease at hatching in chickens vaccinated as embryos with the turkey herpes virus. Avian. Dis. 26:134-149.
Singh U, Devaraj S and Jialal I. (2005). Vitamin E, oxidative stress, and inflammation. Annu. Rev. Nutr. 25:151-174.
Snedecor GW and Cochran WG. (1980). Statistical methods. Iowa State University Press. Iowa.
Surai PF, Noble RC and Speake BK. (1999). Relationship between vitamin E content and susceptibility to lipid peroxidation in tissues of the newly hatched chick. Br. Poult. Sci. 40:406-410.
Theodoratou E, McNeill G, Cetnarskyj R, Farrington SM, Tenesa A, Barnetson R, Porteous M, Dunlop M. and Campbell H. (2007). Dietary fattyacids and colorectal cancer: a case-control study. Am. J. Epidemiol. 166:181-195.
Weiss FG and Scott ML. (1979).Effect of dietary fat and total energy upon plasma cholesterol and other parameters in chickens. J. Nut. 109: 693-701.
Yasser Jamal Jameel, Ali Mahdi Sahib (2014). Effect of In ovo injection with Newcastle Disease Vaccine, Multivitamins AD3E, and Omega-3 on Carcass Characteristics of Broilers. Mirror of Research in Veterinary Sciences and animals. MRSVA. 3 (1), 21-29.