INTRODUCTION
The bursa of Fabricius (BF) is a lymphoid organ, of lympho-epithelial structure. It is a site of B lymphocyte repertoire differentiation and maturation, located in the dorsal terminal part of the cloacae of the birds (Toivanen, et al 1987; Alloui & Sellaoui 2012).
The BF is the essential (primary) target of Gumboro disease virus (IBDV). This disease manifests as acute and subclinical forms in chicks of age 0-3 weeks with immunosuppression or also in clinical form depending on the age of the bird (Sellaoui, et al 2012). The chicks become anorectic, reluctant to move, and show ruffled feathers with watery diarrhea, trembling and severe prostration. The lesions characteristics of the disease include dehydration of the muscles with ecchymotic hemorrhages, enlargement, and orange discoloration of kidneys (Brugere-Picoux & Vaillancourt 2015). The BF becomes enlarged and shows pale yellow discoloration. Intra-follicular hemorrhages may be found and pin point hemorrhages on the skeletal muscles are usually prominent (Madej, et al 2012).
The IBD virus (IBDV) spread in the bursa, by deteriorating its lymphoid structure and causing lesions of different importance, depending on the strains and the immunizing state of the affected birds (Jungbaeck & Nutolo 2001; Juranova, et al 2001). These lesions generally induce an immunosupression which results in the appearance of secondary infections and weak production performances. Typical histopathological lesions of BF have been described by Mazieriegos, et al (1990); Ingrao, et al (2013); Madej, et al (2012); that included mild to severe lymphoid depletion in bursal follicles, follicular atrophy, cystic formation of follicles, and bursal hemorrhage.
This article describes the anatomical and histopathological structure of the chicken BF with IBD compared to healthy ones.
MATERIAL AND METHODS
This histopathological study was carried out in Batna region, located in eastern Algeria. It is a region where poultry farming is very developed. IBD outbreaks are very recurrent. The healthy bursa were taken from ISA chickens strains, aged between 3 and 5 weeks, and bred in a poultry farm of Batna area during slaughtering. Concerning chicken affected by IBD, their BF, were collected after autopsy in a poultry farm of the same area. After collecting, the samples were immediately fixed by formalin (10%) and brought to the anatomopathological laboratory of the Veterinary Institute (Batna University 1) . The preparation of the slides was carried out according to the classical anatomopathologyc method described by Campbell, 1995. On totally, 30 BF were subject of an anatomic and histopathological study. Using a standard microscope Axioskop 20, various slides were observed with various magnifications (H and E x10, H and E x40 and H and E x100).
RESULTS AND DISCUSSION
Physiological and anatomical aspect of BF
The examination of the slides of the healthy bursa revealed the physiological architecture of the organ. The BF has the shape of a chestnut with a sacculated form (Figure 1).
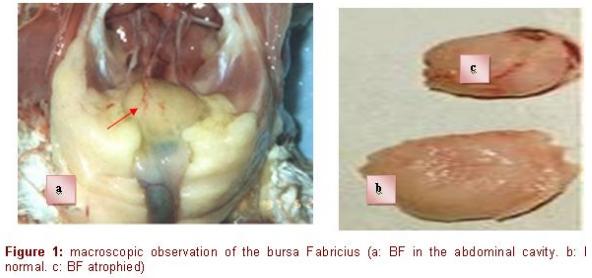
It is a median impair organ, blank at the posterior face of the final intestine with a series of tissue fringes surrounding a central lumen of "spangled" form in continuity with the cloacae cavity (Cazaban, et al 2015; Olah & Vervelde, 2012). The size and the number of the fringes increase with age. Each fringe is made up of several follicles. Their number in a mature bursa is about 8000 to 12000 follicles (Bach, 1979). The follicle is the histological, functional, immunological and pathological unit of BF (Figure 2). The tunica serosa, the tunica muscularis and the tunica mucosa form the wall of the bursa, which is in continuity with that of the intestine. The tunica serosa of the bursa is not very thick and it is associated to smooth muscular fibers distributed into all directions. The tunica muscularis is located under the tunica serosa, made of smooth muscular fibers. The folded tunica mucosa is covered with a pseudostratified epithelium, made up, like that of the intestine, of cylindrical cells, but deprived of mucous cells (Figure 2).
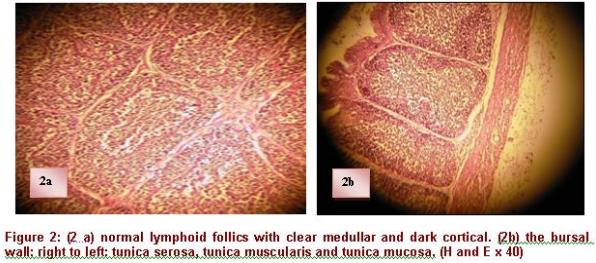
The follicles are located under the lamina propria immediately under the epithelium of coating. Each follicle consists of a lighter medulla and a darker cortex (Figure 3). The medulla contains reticulo-epithelial cells which form a continuous surface with its periphery and come in contact with the epithelium. These cells form a loose network of support, whose spaces are infiltrated by B cells and BSDC (bursal secreting dendritic cells), macrophages, granulocytes, lymphoblast's and some plasmocytes. The follicular cortex develops around the medulla, primarily made up of small lymphocytes and plasmocytes. It is generally formed during the ontogenesis after the connection between the bursal and intestinal lumen is established, generally around the hatching (Nagy & Olah 2000).
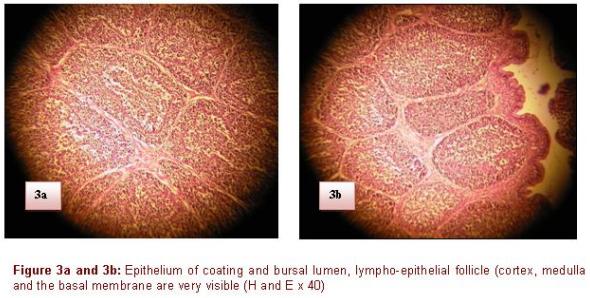
Contrary to the thymus where there is no limit between the cortex and the medulla, it exists in the BF, a basal membrane of 100 to 140 nm separating the medulla of the cortex (Olah & Vervelde 2012). On microphotography's, this one presents a homogeneous central zone measuring approximately 15 nm, bordered on its two faces by glycoprotein amorphous layer. This membrane is not absolutely impermeable and allows the exchanges between the two zones of the lympho-epithelial follicle (Mahgoub, 2012). There is a dense network of lymphatic vessels in the bursa, at the outside o the follicles, not inside. These vessels contain a great number of lymphoid cells, the flux is efferent, they are implied in the transport of cells starting from the bursa. Each follicle has its own provisioning of blood. Rich capillaries surround the medullar area of the follicle which is free from vessels; contrary to the medulla, the vascularization of the cortex is rich in various components of the extracellular matrix, which is used as support for the cellular migration.
The cortico-medullar epithelial cells (CME) form crypts which probably contain the precursors of BSDC. Several histological and functional data suggest that the cells contained in the cortico-medullar crypts are not of hematopoietic origin. They proliferate quickly inside the crypts and when these last open in the medulla, they are released and differentiated to BSDC.
Pathological aspect
The BF in the affected birds aged 3-5 weeks were found showing significant lesions, which were necropsied with different atrophic changes (Raji, et al 2017). These observations are in accordance with those reported by Islam & Samad (2004); Jungbaeck & Nutolo (2001).
When the targeted cells are mainly or exclusively lymphoid cells, the infection with the IBDV is followed by an immunodepression which importance is related to the virulence of the causal agent, the pressure of the infection and the presence or the absence of a preliminary immunity (Mazieriegos, et al 1990). The virus multiplies in BF, by deteriorating its lymphoid structure (Pantin-Jackwood & Brown 2003).
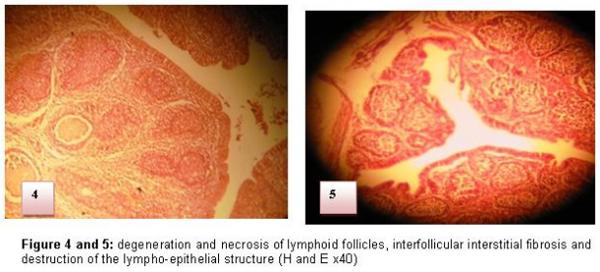
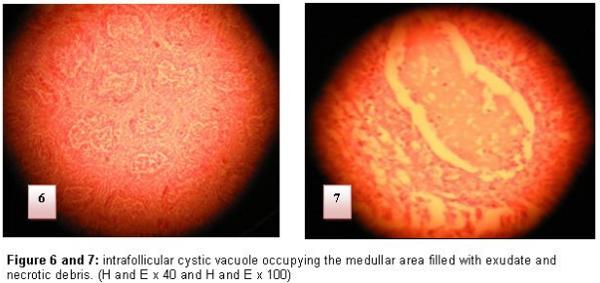
The attack of BF is translated at the beginning by the inflammation, an oedema and an infiltration by the heterophile polynuclears and the macrophages then intervenes degeneration and necroses of medullar lymphocytes of some follicles of the BF. The medulla constitutes the starting point of the lesions changes (Figure 4 and 5). Three to four days later, the medulla of the affected follicles does not contain any more lymphocytes and it is invaded completely by reticular cells. The hypertrophy of the inter-follicular conjunctive tissue and the oedema resulting from the inflammatory reaction lead to a marked hypertrophy of BF. (Figure 6 and 7)
At the end of five to six days, the lesions extend to all cortical and medullar follicles, where only some pycnotic lymphocytes persist. The hypertrophy of interstitial conjunctive tissue continues to be accentuated.
This inflammation disappears then to leave place to cystic vacuoles in the medullar zone, then the bursa is gradually atrophied. The cellular regressive, degenerative and necrotic lesions of the lymphoid cells with lymphocyte depletion of the organ are the consequence of an intracellular cycle of viral replication. From the eighth day, the weight of the bursa is reduced from 1/3 to 1/6 of the standard weight.
The presence of cystic cavities along with the cellular accumulation in the bursal follicles was also reported by Khan, et al 2009 ; whereas Lukert & Saif 2003, reported bursal lesions in vaccinated chickens, which were histopathologically characterized as either normal follicles with or without mild to moderate lymphoid depletion and without follicular atrophy or with the development of cystic follicles
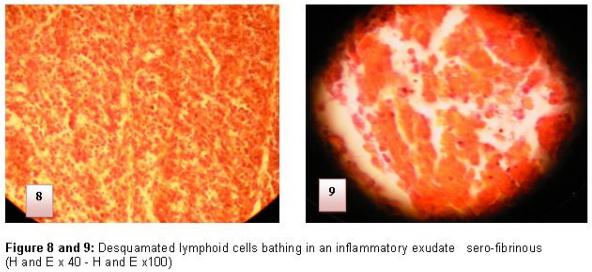
The reversibility of the histological lesions of the BF depends on the importance of the reticulo-histiocytair system destruction. At the end of the evolution, and depending on the strains and the immunizing state of the affected bird, there can be a deterioration of the lymphoid structure and histological architecture of the BF (4) (Figure 8 and 9).
CONCLUSION
The bursa of Fabricius is the support of humoral immunity; its histology utilizes remarkable specific interactions between the epithelial component, the reticular component, the dendritic cells and the lymphoid cells, forming a special microenvironment for the good development and operation of the organ. The follicles attack and the tissue destruction caused by infectious bursal disease virus (IBDV) are behind the immunodepression and bad performances of production. This study enabled us to make histological slides of a good quality, which can contribute and add value to the pedagogical training of students in avian pathology.
This article was originally published in Revista electrónica de Veterinaria. 2017 Volumen 18 Nº 9 - http://www.veterinaria.org/revistas/redvet/n090917.html.