Absorption of selenium by developing chick embryos during incubation
Discovered by Berzelius (1818) in 1817, selenium (Se) is a required nutrient in the diets of poultry (NRC, 1994). Rotruck et al. (1973) identified the element as being required for the functioning of the enzyme Se-dependent glutathione peroxidase (GSH-Px), which has a major role in antioxidant metabolism. Although Se itself is not an antioxidant, as the functional part of the GSH-Px enzyme, Se plays a major role in membrane protection by preventing peroxidation. Provision of Se in commercial poultry diets is not a trivial matter, as it can be toxic even at levels of inclusion as low as 5 ppm (Ort and Latshaw, 1978).
Selenium inclusion in eggs and developing embryos
There is evidence suggesting that the form of Se provided in the diet affects the degree to which it is absorbed by chickens and subsequently deposited in eggs (Cantor et al., 1975a, 1975b; Latshaw and Osman, 1975; Latshaw and Biggert, 1981). A common source of Se for poultry diets has been the inclusion of Se as sodium selenite (Na2SeO3) in the feed.
This product has several disadvantages, including its high degree of toxicity and high density, which make production of feed more difficult. Nevertheless, selenite has been favored as a principal Se source, primarily because of its low cost, and (at least in the US) because until 2000, the US Food and Drug Administration only permitted the use of sodium selenite and sodium selenate as Se supplements for animals. Selenium in the form of Se-yeast, a commercial preparation, contains a large amount of Se as selenomethionine, the Se analog of methionine (Kelly and Power, 1995).
Selenium yeast (Sel-PlexTM Alltech, Inc.) contains 1000 ppm Se and is produced by the fermentation of yeast (Saccharomyces cerevisiae) in a high Se medium. Researchers have shown that Se is deposited in greater amounts in eggs and tissues when Se-yeast is used as the Se source, as opposed to when selenite is used (Moksnes, 1983; Moksnes and Norheim, 1982, 1986).
Published reports note that Se has an effect on hatchability of fertile chicken eggs (Poley et al., 1941; Cantor and Scott, 1974; Latshaw and Osman, 1974). Because both vitamin E and Se have antioxidant functions, there is a close association between the two nutrients.
In periods of Se deficiency the provision of supplemental Se is associated with increased glutathione peroxidase (GSH-Px) activity (Omaye and Tappel, 1974; Hassan, 1986). Surai (2000) reported the sparing effect that Se had on the requirement for vitamin E in the developing chick embryo. This sparing effect would presumably be due to increased GSH-Px activity resulting in increased antioxidant capacity, which would lower the requirement for vitamin E.
Because antioxidant function is important in the developing chick embryo (Hamilton, 1965), and Se affects the activity of GSH-Px, an antioxidant enzyme, data describing the movement of Se within the egg during development would facilitate a clearer understanding of the role of Se in antioxidant defense.
Do different forms and levels of dietary Se affect the level of Se in the developing embryo, as they do in the infertile egg? The amount and timing of Se uptake by the embryo during incubation was hitherto largely unknown. This research describes relationships between the form of dietary Se and its uptake by the embryo, as well as relating the timing of Se uptake to events in embryo development that would presumably require Se.
This research does not attempt to directly address the biological and biochemical functions of Se in metabolism; for this information the reader is instead referred to several comprehensive reviews on the subject (NRC, 1983; Combs and Combs, 1986; Levander, 1986; Sunde, 1997).
THE DEPOSITION OF SELENIUM INTO THE EGG
The eggs of avian species must support life independently for the period of incubation. As a result, birds must incorporate all the necessary nutrients to support the development of the embryo into the egg before it is laid (White, 1991). Because Se is an essential nutrient, it must be incorporated into the egg. The hen’s nutritional status affects the amount of nutrients available during egg formation, and as a result profoundly influences the amount of Se inclusion in the egg (NRC, 1983).
In general, Se is found in greatest concentration in the yolk (Latshaw, 1975; Latshaw and Osman, 1975; Latshaw and Biggert, 1981; Swanson, 1987; Paton et al., 2000), although the specific form of Se in the maternal diet has a large influence as to where the Se is deposited in the egg.
According to Latshaw (1975), Latshaw and Osman (1975) and Latshaw and Biggert (1981), the white of the egg is the site that tended to accumulate more Se if the source of the mineral was organic, while the yolk tended to accumulate more Se if the maternal Se source was inorganic. They stated that organic Se included Se supplied by natural plant sources and additions of synthetic selenomethionine, while inorganic Se was supplied by sodium selenite.
A review of available published research would suggest that increasing dietary Se increases the level of Se in the egg, regardless of the form in which Se is supplied. These data demonstrate that egg Se concentration is dependent on Se source and increases with increasing Se level of the maternal diet. Egg Se concentration plateaus when the dietary Se source is selenite but continues to rise to some threshold beyond that of selenite when the Se source is organic.
Evidence for partitioning of Se into the yolk and white of the egg is clearly presented in the composite graph shown in Figure 1. Values for whole egg, egg yolk and egg white Se concentration were obtained or calculated from 20 published papers and plotted according to the source and inclusion level of Se (Cantor and Scott, 1974; Latshaw and Osman, 1974, 1975; Latshaw, 1975, 1986; Kääntee and Kurkela, 1980; Cantor, 1981; Latshaw and Biggert, 1981; Martello and Latshaw, 1982; Moksnes and Norheim, 1982; Kääntee et al. , 1982; Moksnes, 1983; Swanson et al., 1983; Laws et al., 1986; Swanson, 1987; Robberecht et al., 1987; Davis and Fear, 1996; Paton et al., 1998, 2000; Cantor et al., 2000).
Egg Se concentrations were plotted on the ordinate and feed Se levels on the abscissa. To demonstrate relative differences between the different components of the egg, each of the graphs used the same scale. Using linear and non-linear regression procedures of SAS (PROC NLIN), the best fitted line was selected (SAS Institute Inc., 1999), in all cases this was a second order polynomial.
The relationship between whole egg Se content and dietary Se source and level is seen in the top graph in Figure 1. When feed contains Se from organic sources, which include naturally occurring Se in feed ingredients and any added selenomethionine, the whole egg Se increases as the feed Se level increases. The relationship is curvilinear and does not plateau to an asymptote within the feed Se range of 0 to 0.5 ppm. When the feed Se is primarily provided by sodium selenite, whole egg Se plateaus at about 0.2 ppm and selenite Se supplementation beyond 0.4 ppm has little effect on whole egg Se concentration.
The response of yolk Se to dietary Se source and level (middle graph in Figure 1) is similar to whole egg Se, except that the response to dietary selenite Se results in a plateau at a higher concentration of yolk Se (0.55 ppm). The response to organic sources of Se rises with dietary Se, as in whole egg Se, except that the slope of the fitted line is steeper for yolk Se than for whole egg Se.
In egg white, effects of changing dietary Se source and level differ from those observed in whole egg and yolk Se accumulation (bottom graph in Figure 1). Selenite Se is not incorporated into the egg albumen to the same extent as Se from organic sources. However, organic Se is incorporated into egg albumen to a lesser extent than it is into egg yolk. An explanation for this may lie in the way the egg is formed. The egg yolk is the major source of minerals (except for calcium) for the developing chick and as such readily accumulates the necessary minerals for inclusion into the egg.
Biologically it would be detrimental for required minerals to be unavailable to the ovary for inclusion into the egg. Latshaw (1975), Latshaw and Osman (1975) and Latshaw and Biggert (1981) reported that Se supplied by organic sources or selenomethionine was more available to the egg white, while Se from selenite was more available to the egg yolk.
The review of the data presented in Figure 1 supports this statement only in part. It would seem that Se from organic forms is available to the egg white but not more available than it is to egg yolk. The slope of the linear section of organic Se in egg white is approximately 0.67, whereas the same slope for egg yolk is 1.9. Thus, Se in either form is more available to the egg yolk than it is to egg white. This makes sense in that the yolk, and hence the ovary, is the major supplier of minerals to the developing egg.
Selenium incorporated into the albumen would simply be attributed to substitution of methionine for selenomethionine. In summary, it would appear that different metabolic Se reserves are affected by Se source and are distinct from one another (Moksnes and Norheim, 1986).
However, different Se pools can and do contribute to supply a common Se requirement, but they do so at different rates and efficiencies.
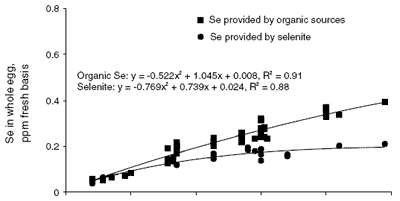 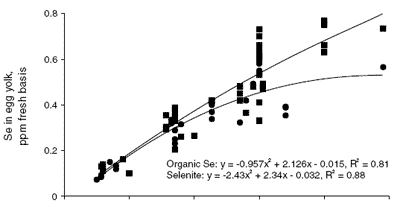 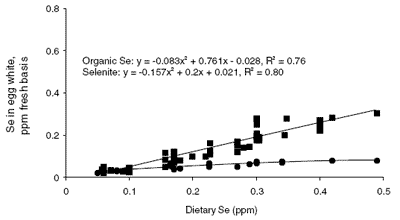 |
| Figure 1.Composite graph of whole egg, egg yolk and egg white Se as affected by dietary Se source and level (y is the resultant egg Se content (ppm fresh basis) and x is the Se content of the maternal diet). |
Selenium inclusion into the developing embryo
Selenium is involved in the fertility of poultry and the hatchability of fertile eggs. Selenium supplementation of diets deficient in Se has improved both fertility and hatchability (Poley et al., 1941; Cantor and Scott, 1974; Latshaw and Osman,1974; Combs, 1994; Klecker et al., 1999).
As discussed above, Se content of the diet also influences the Se content of eggs. These two issues may be related in some manner. If the movement of Se from the extra-embryonic material to the embryo during incubation were characterized, then researchers would better understand the role of Se in the developing embryo. However no published data exist that quantify the relationship between the dietary Se content of the hen’s feed and the amount of Se incorporated into the chick embryo as it develops.
The research presented in this paper describes the relationship between the source and level of Se in the hen’s diet and the transfer of Se from the extraembryonic material to the developing embryo.
Two experiments were conducted. The first trial identified the period of incubation that was most important as far as Se movement in the egg was concerned. This was achieved by measuring the amount and concentration of Se in the extraembryonic and embryonic portions of the egg at five specific times during incubation. The second trial produced data that illustrated daily Se movement during the specific period of incubation identified in the first trial.
SELENIUM MOVEMENT IN THE EGG THROUGHOUT INCUBATION
A flock of 120 laying hens was fed a low Se diet (negative control diet ~ 0.06 ppm Se) for 16 weeks.
Following this they received one of seven experimental diets for 42 days (the experimental period). The seven test diets were designed to form a factorial arrangement of two Se sources (sodium selenite or Sel-PlexTM Se-yeast) added at three levels of dietary Se (0.1, 0.2 and 0.3 ppm), plus a negative control diet with no added Se. The assayed Se levels reflected the low amount of Se present in the negative control diet plus the additional Se provided by the supplement (Table 1).
| Table 1. Depletion diet and experimental feed Se concentration (Experiment 1)1. |
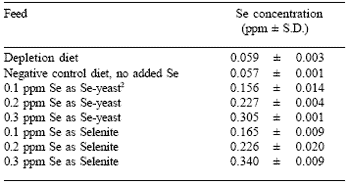 |
| 1 Values are means ± standard deviation of three assays reported on as-is basis. 2 Sel-PlexTM, Alltech Inc. |
On days 30, 31 and 38 of the experimental period the hens were artificially inseminated and fertile eggs were subsequently collected. The collected eggs were incubated for 0, 5, 10, 15 or 20 days. After removal from the incubator (or storage) the eggs were separated into egg yolk and white (eggs that were not incubated) or embryonic and extraembryonic material (eggs incubated for 5, 10, 15 and 20 days).
The embryonic portion of the egg was just the physical embryo, while the extraembryonic portion of the egg was comprised of the albumen, the amniotic fluid and membrane, the yolk and the yolk sac membrane. The various portions of the egg were then homogenized and assayed for Se.
Selenium content of the egg
There were no differences in hen feed intake or average egg production during the experimental period. There were also no differences in egg weight due to dietary treatment before or after incubation.
For eggs that were not incubated, Se content was significantly affected by dietary treatment. The results were in good agreement with the data in Figure 1. Addition of any form of Se to the diet elevated whole egg, egg yolk and egg white Se.
Selenium from yeast increased egg. Se to levels above those observed for eggs from hens fed diets containing sodium selenite. The whole egg Se levels are presented in Figure 2.
Selenium content of the developing embryo
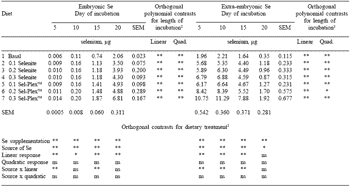
As expected, total egg Se content for incubated eggs did not differ among incubation times. Maternal dietary Se had a significant impact on Se content of the total egg, embryo and extra-embryonic portions of eggs incubated for 5, 10, 15 or 20 days (Figure 3). The actual amounts of Se in the embryonic and extra-embryonic portions are presented in Table 2.
As expected, embryos from hens fed diets containing any Se supplementation contained greater concentrations of Se than embryos from the negative control. Embryos from hens given Se-yeast contained significantly more Se than did the embryos from hens given selenite.
In every instance, the response to increasing dietary Se was linear. A greater increase in embryo Se concentration was achieved for each incremental amount of dietary Se addition when the source was Se-yeast than when it was sodium selenite. There were significant linear increases in embryo Se concentration during incubation. The longer the period of incubation, the greater the concentration of embryo Se. The most obvious increase was between day 10 and 15 when average embryo Se increased by 0.05 ppm.
These observations may be related to reports in the literature. Increases in the activity of GSH-Px in the developing chick would require additional Se in the diet of the hen (Omaye and Tappel, 1974; Combs and Scott, 1979; Hassan, 1986; Surai, 2000).
Surai (1999) and Surai et al. (1997) reported that the liver activity of GSH-Px in the developing chick embryo rises quickly during days 10 to 15 of incubation. Together these findings dictate that additional Se must be supplied to the embryo during this period of incubation; and the data reported in the present study confirm this relationship.
Linear increases in GSH-Px activity in the chick liver between days 8 and 18 of embryonic development were also reported by Wilson et al. (1992), but enzyme activity was assessed only on days 8, 12 and 18. In chick embryos, the development of glial cells in the brain after day 10 of incubation requires increased activity of GSH-Px (Wilson et al., 1992).
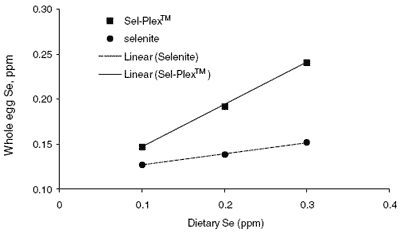 |
| Figure 2.Whole egg Se as affected by dietary Se content. Lines have been fitted to show the difference in slope between the two Se sources. |
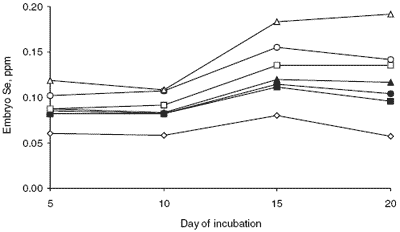 |
| Figure 3. Embryo Se concentration during incubation as affected by dietary Se source and level. |
During the same period there are also increases in the activities of catalase and SOD in the liver of developing embryos. Increases in the concentration of α-tocopherol in the liver of developing chick embryos after day 13 were reported by Surai et al. (1996) and Noble et al. (1993). This presumably reflects an increased antioxidant status which might be associated with additional requirements for GSHPx and hence Se.
This body of research suggests that metabolic processes during this stage of embryonic development are particularly vulnerable to oxidative destruction and the embryo establishes a level of antioxidant protection large enough to prevent significant damage. The data observed in this trial support other published reports and provide firm evidence that Se provision to the embryo during this stage of development is critical.
Selenium content of the extra-embryonic material
Extra-embryonic Se concentration is also affected by the amount and source of Se in the maternal diet, and it changes throughout incubation (Figure 4). The differences in extra-embryonic Se are presented in Table 2, and serve to indicate total amounts of Se observed.
The changes in the extraembryonic Se concentration throughout incubation were analyzed. There were both significant linear and quadratic responses in extra-embryonic Se level to length of incubation. The extra-embryonic Se concentration increased sharply after day 15 of incubation. While the increase was notable, the elevated concentration probably has more to do with the fact that on day 20 of incubation there is very little extra-embryonic material left, and the actual amounts of Se, while concentrated, are small as indicated in Table 2.
SELENIUM MOVEMENT IN THE EGG DURING DAYS 10 TO 15 OF INCUBATION
In the second study the objective was to determine if there was a specific time point in the period of incubation identified in the first study (10 to 15 days) that was very important for embryo Se status or metabolism. If a specific time point was identified, the study aimed to relate this to other aspects of embryonic ontogenesis and metabolism.
The second study was similar to the first in that fertile eggs were collected from hens fed different sources of Se at two levels. This time, the period of incubation focused on development of the embryo between days 10 and 15. The study used 96 laying hens that had been fed a Se depletion diet for 12 weeks. Subsequently the birds were fed one of four experimental diets for 40 days. The four test diets formed a factorial arrangement of two Se sources (sodium selenite or Se-yeast) at two levels of Se supplementation (0.1 and 0.3 ppm additional Se).
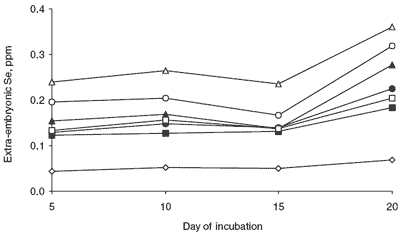 |
| Figure 4. Extra-embryonic Se concentration during incubation as affected by dietary Se source and level. |
The assayed Se levels of the diets are given in Table 3. The fertile eggs were then incubated for 10 to 15 days. The contents were then separated into embryonic and extra-embryonic portions and assayed for Se. One of the objectives of the trial was to investigate further the increase in embryo Se during days 10 to 15 of incubation.
The broken stick or broken line method used by Robbins (1986) to estimate nutrient requirements from growth data was modified and used to describe when, and at what level, Se concentration in the embryo began to increase. The modeling was completed using the PROC NLIN procedure of SAS (SAS Institute Inc., 1999), which provided estimates of the breakpoint and slopes in the two models.
| Table 3.Depletion diet and experimental feed Se concentration (Experiment 2)1. |
 |
| 1 Values are means ± standard deviation of three assays reported on an “As-is” basis. |
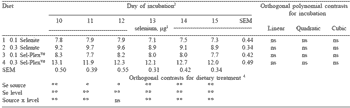
Selenium content of the developing embryo Hen production performance was unaffected by dietary treatment. As expected, there was no loss or gain of Se during the incubation period. There were however differences observed in egg Se content among the four dietary treatments. When Se was provided by Se-yeast, total egg Se was higher than when sodium selenite was added to the diet. Higher amounts of dietary Se elevated total egg Se at all stages of incubation, in agreement with earlier observations.
There was also a significant interaction between Se source and level for all but one day of the incubation period. This indicated that the response to additional Se supplementation was different between the two Se sources. The response in total egg Se due to feeding an additional 0.2 ppm Se was greater when the Se source was Se-yeast than when Se was provided as sodium selenite.
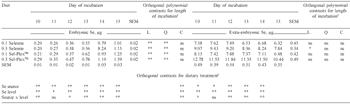
Figure 5 illustrates the change in embryo Se concentration as incubation proceeds. As was the case in the previous study, there were increases in embryo Se concentration during this period.
Throughout the five-day period, Se concentration in embryos from hens fed the diet containing 0.3 ppm Se-yeast was greatest, followed by embryos from hens fed the 0.1 ppm Se-yeast diet, then the 0.3 ppm selenite Se diet and finally the 0.1 ppm selenite Se diet.
Contrasts performed to investigate differences in Se source, level (0.1 or 0.3 ppm) and a source by level interaction were all significant (at P≤0.01) in every period of incubation. It is evident that both Se source and dietary level have a large influence on the embryonic Se concentration during this stage of development.
In embryos from individual dietary treatments, the effect of incubation and embryonic development on embryonic Se concentration was evident. Selenium concentration in embryos increased in a quadratic manner as incubation progressed. The embryonic Se concentration at the end of the whole incubation period (day 10 – 15) was significantly higher than that in the beginning.
Four lines were fitted to the data using the broken stick approach of Robbins (1986) (Figure 5). In all four cases the regression models fitted were significant (P≤0.01) and described most of the variation in the data. All the models indicated that the breakpoint (indicated by the dashed line in Figure 5), the point where Se concentration of the embryo begins to increase, was near 12.25 days of incubation.
The observation that embryo Se concentration begins to increase at day 12.25 irrespective of source or level of Se inclusion in the maternal diet was the most significant finding in this study. Because there were differences among total egg concentrations and amounts of Se in the incubated eggs, it can also be stated that this observed point of increase is independent of the source of Se and the amount of Se contained in the developing egg.
This implies that irrespective of the level or source of Se available to the embryo, the developing chick has an elevated requirement for Se beginning on or about day 12 of incubation. As a result, the embryo begins to absorb additional Se to support these requirements.
There is substantial evidence in the literature to support these findings. When the chick embryo is 12.25 days old, it is considered to be at stage 38 of embryonic development (Hamilton, 1965).
Physically the chick is easily identifiable as an avian and it has begun to develop feathers that appear in feather tracts. While the embryo has absorbed water from the albumen up to this stage, very little solid material has been absorbed from the egg white up to the 13th day (Freeman and Vince, 1974).
Romanoff (1960) reported that on about day 13 there is a major movement of albumen, the seroamniotic division is ruptured and the albumen flows into the amniotic cavity. Transfer of the albumen is rapid and 70% moves into the amniotic cavity within one day (Wise et al., 1964).
During this time, some of the albumen moves into the yolk sac through the yolk sac umbilicus (McIndoe, 1960) and appears in the yolk. The proteins and solids become available to the embryo when the amniotic fluid is incorporated into the developing chick. This begins on the 12th day, but activity increases substantially after day 14 (Romanoff, 1960).
If the incorporation of the albumen starts on day 12, then any Se in the albumen would become available at this time. This may partially explain the increase in embryo Se concentration after day 12.25. As discussed above, the albumen contains little Se when the dietary Se source is selenite and it is probable that some of the increase in embryo Se would be due to the incorporation of albumen into the embryo via the yolk umbilicus. However, this occurrence probably cannot account for the entire response seen in Figure 5.
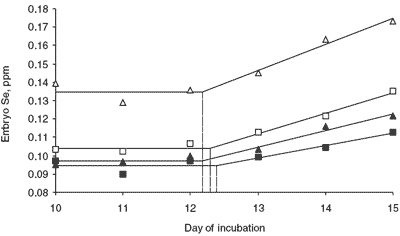 |
| Figure 5.Embryonic Se concentration during incubation as affected by dietary Se source and level. |
It would appear that the absorption of components from the yolk is not uniform. Minerals and nitrogencontaining compounds are absorbed throughout incubation, but lipid material is not (Romanoff, 1960; Hamilton, 1965; Freeman and Vince, 1974). The absorption of yolk lipids (triglycerides and phospholipids) by the embryo increases dramatically from day 13 onwards (Freeman and Vince, 1974; Noble and Cocchi, 1990; Surai, 1999).
It was reported by Noble and Moore (1964) that almost the entire lipid content of the yolk is absorbed and metabolized by the chick during the last seven days of incubation. As evidence of this activity, Noble and Shand (1985) reported the increased activity of liver Δ9- and Δ6-desaturase enzymes and increased production of their lipid metabolites. The Δ6-desaturase enzymes are responsible for the first step in converting linoleic acid to arachidonic acid.
There is little arachidonic acid in the yolk, but embryonic requirements for the fatty acid are high due to its extensive incorporation into membrane phospholipids (Whitehead, 1991). The internalization of yolk lipids is associated with increases in embryo liver antioxidant activity, specifically GSH-Px, SOD and catalase (Surai et al., 1997; Surai, 1999; Wilson et al., 1992), because yolk lipids, and particularly PUFA, are very susceptible to oxidation.
Noble and Shand (1985) reported an increase in vitamin E uptake by chick and poult embryos at this stage, and large increases of the vitamin have been previously detected in the liver (Noble et al., 1993; Gaal et al., 1995; Surai et al., 1996; Surai et al., 1999). Increased GSH-Px activity requires an increased supply of Se (Omaye and Tappel, 1974; Combs and Scott, 1979; Hassan, 1986; Toyoda et al., 1990; Surai, 2000).
Thus, it would appear that the increase in embryonic Se concentration is associated with increased GSH-Px activity. The increase in GSH-Px activity would be required due to a general up-regulation of antioxidant activity, which would be due to an increased state of lipid absorption and metabolism. All these processes begin around day 12 or 13 in the developing chick.
On day 12 of incubation, the spleen begins to function as a hematopoietic organ and produces primitive lymphoid cells that differentiate into leucocytes and erythrocytes (Olson, 1952). Up to this stage, the yolk sac membrane (YSM) has produced most of the blood for the embryo (Hamilton, 1965). The development of erythrocytes would require additional synthesis of GSH-Px, because this enzyme is required in erythrocytes (Omaye and Tappel, 1974).
A deficiency of Se has been shown to influence the level of GSH-Px mRNA during transcriptional processing (Toyoda et al. , 1990), which implies that Se would be required in early stages of GSH-Px synthesis. From this discussion, it is clear that Se would be required for blood cell synthesis in the embryo after day 13 of incubation.
The adenohypophyseal-thyroid axis (embryonic thyroid) of the chick starts to become functional on day 11.5 of incubation (Thommes et al., 1977), and the thyroid of the chick begins to convert thyroxine (T4) to 3,3',5-triiodothyronine (T3), the active form of the thyroid hormone (Arthur, 1997; Sechman and Bobek, 1988).
The conversion of T4 to T3 involves a group of enzymes called the iodothyronine deiodinases, of which there are three types. All three types are Se dependant to some extent, but type I (IDI) is expressed in the liver and has been shown by Beckett et al. (1987, 1989) to require Se. As a result, the enzyme activity is decreased when Se is deficient. The initiation of embryo thyroid function would require the iodothyronine deiodinases.
Because the enzymes contain Se, it is logical to assume that the embryo would require additional Se for thyroid function beginning on day 11.5 of incubation.
Total embryo Se changed significantly during incubation (Table 5). Each day of incubation produced embryos with significantly more Se than the day before. In all cases the increase was also quadratic as incubation proceeded. This observation is due to an increase in embryonic Se concentration (Figure 5) and increases in embryo size.
The increased Se in the embryos from hens fed diets containing 0.3 ppm Se (vs 0.1 ppm Se) would support the data reported by Combs and Scott (1979), Hassan (1986) and Surai (2000), who showed that when maternal diet Se content increases, there is a concomitant rise in chick Se level at hatching. No published data have shown the difference between Se sources and levels in the newly hatched chick.
However, because the chick absorbs most of the egg contents, it is logical to state that organic sources of Se would elevate chick levels of Se. Selenium content of the extra-embryonic material
Extra-embryonic Se levels decreased numerically as the eggs developed during incubation, and this decrease was significant for the 0.3 ppm selenite Se treatment (Table 5). Although changes in the amounts of extra-embryonic Se within treatment were not significant between days 10 and 15, a significant decrease in total extra-embryonic Se during days 0 to 20 would be expected based on the results of Experiment 1. In every incubation period, effects of source and level were significant.
With the exception of day 12, the interaction of Se source and level was significant during the length of incubation. These findings concur with those for total embryonic Se. As the dietary Se increased, so did egg Se and hence extra-embryonic Se. Selenium from Se-yeast increased Se levels in the extraembryonic material to a greater extent than Se provided by selenite. The higher the dietary Se in the diet, the higher the extra-embryonic Se in the developing egg.
| Conclusions The development of the chick embryo requires an adequate supply of nutrients during incubation. Because these must be supplied before the egg is laid, there is no opportunity for additional nutrients to be supplied while the embryo is developing. As such, it becomes all the more critical to ensure that nutrients supplied to the hen are readily available to the developing embryo. The case of Se is a good example. The mineral is required for antioxidant protection during development. It is evident from these studies that the chick relies on an adequate supply of Se during specific periods of incubation. Provision of more available forms of Se would better ensure that Se was always in adequate supply. This may promote the emergence of healthier and less nutritionally-stressed chicks at hatch, and possibly reduce mortality for embryos during incubation as well as for chicks during early growth. |
References
Arthur, J.R. 1997. Non-glutathione peroxidase functions of selenium. In: Biotechnology in the Feed Industry: Proceedings of Alltech’s 13th Annual Symposium (T.P. Lyons and K.A. Jacques, eds), Nottinghan University Press, Notthinham, United Kingdom, pp. 143-154.
Beckett, G.J., S.E. Beddows, P.C. Morrice, F. Nicol and J.R. Arthur. 1987. Inhibition of hepatic deiodination of thyroxine caused by Se deficiency in rats. Biochem. J. 248:443-447.
Beckett, G.J., D.A. MacDougall, F. Nicol and J.R. Arthur. 1989. Inhibition of type I and type II iodothyronine deiodinase activity in rat liver, kidney and brain produced by Se deficiency. Biochem. J. 259:887-892.
Berzelius. 1818. Sur deux métaux nouveaux. Annales de Chimie et de Physique. Ser. 2, 7:199- 206.
Cantor, A.H. 1981. Effect of dietary selenium on the concentration of selenium in turkey eggs. Poultry Sci. 60:1094-1096.
Cantor, A.H. and M.L. Scott. 1974. The effect of selenium in the hen’s diet on egg production, hatchability, performance of progeny and selenium concentration in eggs. Poultry Sci. 53:1870-1880.
Cantor, A.H., M.L. Scott and T. Noguchi. 1975a. Biological availability of selenium in feedstuffs and selenium compounds for prevention of exudative diathesis in chicks. J. Nutr. 105:96-105.
Cantor, A.H., M.L. Langevin, T. Noguchi and M.L. Scott. 1975b. Efficiency of selenium in compounds and feedstuffs for the prevention of pancreatic fibrosis in chicks. J. Nutr. 105:106-111.
Cantor, A.H., M.L. Straw, M.J. Ford, A.J. Pescatore and M.K. Dunlap. 2000. Effect of feeding organic selenium in diets of laying hens on egg selenium content. In: Egg Nutrition and Biotechnology (J.S. Sim, S. Nakai and W. Guenter, eds), CAB International, Wallingford, United Kingdom, pp. 473-476.
Combs, Jr., G.F. 1994. Clinical implications of selenium and vitamin E in poultry nutrition. Vet. Clin. Nutr. 1:133-140.
Combs, G.F., Jr. and M.L. Scott. 1979. The selenium needs of laying and breeding hens. Poultry Sci. 58:871-884.
Combs, Jr., G.F. and S.B. Combs. 1986. The Role of Selenium in Nutrition. Academic Press, Inc., Orlando, FL.
Davis, R.H. and J. Fear. 1996. Incorporation of selenium into egg proteins from dietary selenite. Br. Poult. Sci. 37:197-211.
Freeman, B.M. and M.A. Vince. 1974. The Development of the Avian Embryo. Chapman and Hall Ltd., London, United Kingdom.
Gaál, T., M. Mézes, R. C. Noble, J. Dixon and B.K. Speake. 1995. Development of antioxidant capacity in tissues of the chick embryo. Comp. Biochem. Physiol. 112B:711-716.
Hamilton, H.L. 1965. Lillie’s Development of the Chick An Introduction to Embryology. 3rd ed. Holt, Reinhart and Winston Inc., New York, NY.
Hassan, S. 1986. Effect of dietary selenium on the prevention of exudative diathesis in chicks, with special reference to selenium transfer via eggs. J. Vet. Med. A. 33:689-697.
Kääntee, E. and P. Kurkela. 1980. Comparative effects of barley feed and sodium selenite on selenium levels in hen eggs and tissues. J. Sci. Agric. Soc. Finland. 52:357-367.
Kääntee, E., P. Kurkela and K. Jaakkola. 1982. Effects of dietary organic selenium content on fowls, chicks and eggs. J. Sci. Agric. Soc. Finland. 54:113-118.
Kelly, M.P. and R.F. Power. 1995. Fractionation and identification of the major selenium containing compounds in selenized yeast. J. Dairy Sci. 78(Suppl. 1):237.
Klecker, D., L. Zeman, A. Bunešová and V. Šiške. 1999. Effect of organic selenium, zinc, and manganese on reproductive traits of laying hens and cockerels. In: Eggs and Egg Products Quality. Proceedings of 8th European Symposium on the Quality of Eggs and Egg Products. World’s Poultry Science Association, Bologna, Italy, pp. 183-185.
Latshaw, J.D. 1975. Natural and selenite selenium in the hen and the egg. J. Nutr. 105:32-37.
Latshaw, J.D. 1986. Eggs give clues about dietary selenium. In: Poultry Pointers (G. Havenstein, ed), Miscellaneous publication, Cooperative Extension Service, The Ohio State University, Columbus, OH, pp. 1-2.
Latshaw, J. D. and M. Osman. 1974. A selenium and vitamin E responsive condition in the laying hen. Poultry Sci. 53:1704-1708.
Latshaw, J.D. and M. Osman. 1975. Distribution of selenium in egg white and yolk after feeding natural and synthetic selenium compounds. Poultry Sci. 54:1244-1252.
Latshaw, J.D. and M.D. Biggert. 1981. Incorporation of selenium into egg proteins after feeding selenomethionine or sodium selenite. Poultry Sci. 60:1309-1313.
Laws, J.E., J.D. Latshaw and M. Biggert. 1986. Selenium bioavailability in foods and feeds. Nutr. Rep. Int. 33:13-24.
Levander, O.A. 1986. Selenium. In: Trace Elements in Human and Animal Nutrition. Vol. 2 (W. Mertz, ed), Academic Press Inc., San Diego, CA, pp. 209-279.
Martello, M.A. and J.D. Latshaw. 1982. Utilization of dietary selenium as indicated by prevention of selenium deficiency and by retention in eggs. Nutr. Rep. Int. 26:43-50.
McIndoe, W.M. 1960. Changes in the protein content of the yolk during chick embryogenesis. J. Embryol. Exp. Morph. 8:47-53.
Moksnes, K. 1983. Selenium deposition in tissues and eggs of laying hens given surplus of selenium as selenomethionine. Acta Vet. Scand. 24:34-44.
Moksnes, K. and G. Norheim. 1982. Selenium concentrations in tissues and eggs of growing and laying chickens fed sodium selenite at different levels. Acta Vet. Scand. 23:368-379.
Moksnes, K. and G. Norheim. 1986. A comparison of selenomethionine and sodium selenate as a supplement in chicken feeds. Acta Vet. Scand. 27:103-114.
National Research Council. 1983. Selenium in Nutrition. Rev. ed. National Academy Press, Washington D.C.
National Research Council. 1994. Nutrient Requirements of Poultry. 9th rev. ed. National Academy Press, Washington D.C.
Noble, R.C. and J.H. Moore. 1964. Can. J. Biochem. 42:1728-1741.
Noble, R.C. and J.H. Shand. 1985. Unsaturated fatty acid compositional changes and desaturation during the embryonic development of the chicken (Gallus domesticus). Lipids. 20:278-282.
Noble, R.C. and M. Cocchi. 1990. Lipid metabolism and the neonatal chicken. Prog. Lipid Res. 29:107- 140.
Noble, R., M. Cocchi and H.M. Bath. 1993. α- Tocopherol absorption and polyunsaturated fatty acid metabolism in the developing chick embryo. Br. Poult. Sci. 34:815-818.
Olson, Jr., C. 1952. Avian Hematology. In: Diseases of Poultry (H.E. Biester and L.H. Schwarte, eds), The Iowa State College Press, Ames, IA, pp. 71- 91.
Omaye, S.T. and A.L. Tappel. 1974. Effect of dietary selenium on glutathione peroxidase in the chick. J. Nutr. 104:747-753.
Ort, J.F. and J.D. Latshaw. 1978. The toxic level of sodium selenite in the diet of laying chickens. J. Nutr. 108:1114-1120.
Paton, N.D., A.H. Cantor, M.J. Ford, B.T. Slaugh, A.F. Rizvi and P. Karnezos. 1988. Effect of providing organic selenium and chromium as yeast in laying hen diets on nutrient composition of eggs. Poultry Sci. 77(Suppl. 1):11.
Paton, N.D., A.H. Cantor, A.J. Pescatore, M.J. Ford and C.A. Smith. 2000. Effect of dietary selenium source and level of inclusion on selenium content of incubated eggs. Poultry Sci. 79(Suppl. 1):40.
Poley, W.E., W.O. Wilson, A.L. Moxon and J.B. Taylor. 1941. The effect of selenized grains on the rate of growth in chicks. Poultry Sci. 20:171- 179.
Robberecht, H., H. Benemariya, P. van Dael and H Deelstra. 1987. Mineralization procedures and determination of selenium in egg white, egg yolk and shells of different eggs. Belgian J. Food Chem. Biotech. 42:147-153.
Robbins, K.R. 1986. A method, SAS program, and example for fitting the broken-line to growth data. In: Research Report 86-09. The University of Tennessee, Agricultural Experiment Station, Knoxville, TN, pp. 1 – 8.
Romanoff, A.L. 1960. The Avian Embryo Structural and Functional Development. The Macmillian Company, New York, NY.
Rotruck, J.T., A.L. Pope, H.E. Ganther, A.B. Swanson, D.G. Hafeman and W.G. Hoekstra. 1973. Selenium: biochemical role as a component of glutathione peroxidase. Science. 179:588-590.
SAS Institute Inc. 1999. The SAS system for Windows. Release 8.00. SAS Institute Inc., Cary, NC.
Sechman, A. and S. Bobek. 1988. Presence of iodothyronines in the yolk of the hen’s egg. Gen. Comp. Endocrin. 69:99-105.
Sunde, R.A. 1997. Selenium. In: Handbook of Nutritionally Essential Mineral Elements (B.L. O’Dell and R.A. Sunde, eds), Marcel Dekker Inc., New York, NY, pp. 493-556.
Surai, P.F. 1999. Tissue-specific changes in the activities of antioxidant enzymes during the development of the chicken embryo. Br. Poult. Sci. 40:397-405.
Surai, P.F. 2000. Effect of selenium and vitamin E content of the maternal diet on the antioxidant system of the yolk and the developing chick. Br. Poult. Sci. 41:235-243.
Surai, P.F., R.C. Noble and B.K. Speake. 1996. Tissue-specific differences in antioxidant distribution and susceptibility to lipid peroxidation during development of the chick embryo. Biochimica et Biophysica Acta. 1304:1-10.
Surai, P.F., B.K. Speake, R.C. Noble and N.H.C. Sparks. 1997. Antioxidant systems of the developing chicken embryo: glutathione peroxidase. Br. Poult. Sci. 38(Suppl.):S19-S20.
Surai, P.F., N.H.C. Sparks and R.C. Noble. 1999. Antioxidant systems of the avian embryo: tissuespecific accumulation and distribution of vitamin E in the turkey embryo during development. Br. Poult. Sci. 40:458-466.
Swanson, C.A.. 1987. Comparative utilization of selenite, selenomethionine and selenized yeast by the laying hen. Nutr. Res. 7:529-537.
Swanson, C.A., D.C. Reamer, C. Veillon and O.A. Levander. 1983. Intrinsic labeling of chicken products with a stable isotope of selenium (76Se). J. Nutr. 113:793-799.
Thommes, R.C., R.L. Vieth and S. Levasseur. 1977. The effect of hypophysectomy by means of surgical decapitation on thyroid function in the developing chick embryo. I. Plasma thyroxine. Gen. Comp. Endocrinol. 31:29-36.
Toyoda, H., S. Himeno and N. Imura. 1990. Regulation of glutathione peroxidase mRNA level by dietary Se manipulation. Biochimica et Biophysica Acta. 1049:213-215.
White, III, H.B. 1991. Maternal diet, maternal proteins and egg quality. In: Egg Incubation: Its Effects on Embryonic Development in Birds and Reptiles (D.C. Deeming and M.W.J. Ferguson, eds), Cambridge University Press, Cambridge, United Kingdom, pp. 1-5.
Whitehead, C.C. 1991. Nutrition of the breeding bird and developing embryo. In: Avian Incubation (S.G. Tullett, ed), Butterworth-Heinemann, London, United Kingdom, pp. 227-238.
Wilson, J.X., E.M.K. Lui and RF. Del Maestro. 1992. Developmental profiles of antioxidant enzymes and trace metals in chick embryo. Mech. Ageing Dvlp. 65:51-64.
Wise, R.W., B. Ketterer and I.A. Hanse. 1964. Prealbumens of embryonic chick plasma. Comp. Biochem. Physiol. 12:439-443.
Authors: NEIL D. PATON1, AUSTIN H. CANTOR2, ANTHONY J. PESCATORE2, MICHAEL J. FORD2 and CYNTHIA A. SMITH2
1 Akey, Lewisburg, OH, USA
2 University of Kentucky, Lexington, KY, USA








.jpg&w=3840&q=75)