Emergence of Tobramycin Escherichia coli resistance in poultry meat linked to biocides overuse during COVID-19
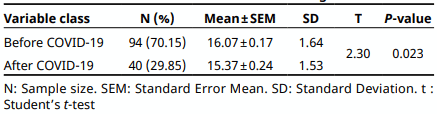
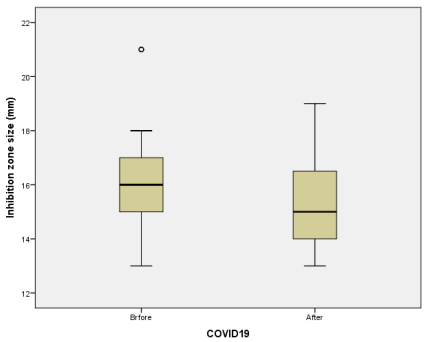
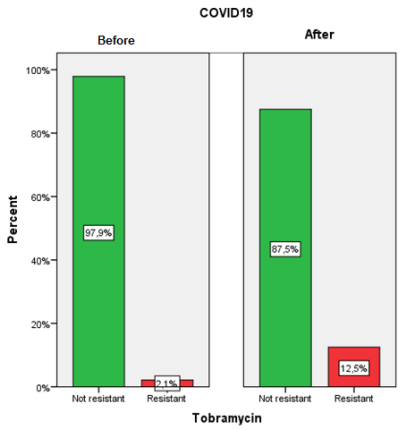
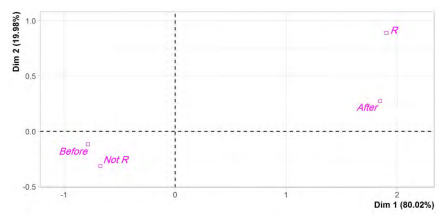
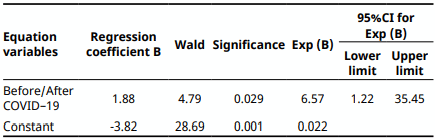
The effect of excessive use of biocides during the COVID-19, on the resistance of Escherichia coli to Tobramycin in poultry, meat was examined in this observational epidemiological study (Before and after COVID–19). Tobramycin E.coli resistant strains isolated from poultry meat before COVID-19 appearance were compared with those isolated after COVID-19 emergence. Univariable analyses were performed using t-test and chi-squared test. Odds ratios and 95% confidence intervals were used for statistically significant risk factor. Multivariate analysis was done with the binary logistic regression to detect an independent predictor, and with the principal component analysis (PCA), to analyze whether the Tobramycin resistance in E. coli was linked with the COVID-19 outbreak. Statistical significance was set at P<0.05. The frequency of Tobramycin E. coli resistant isolates was more important after COVID-19 emergence (12.5%) than before COVID-19 (2.1%). Graphical representation of PCA qualitative variables shows the interfactor relationship. A significant relationship between Tobramycin E.coli resistance and COVID-19 emergence (P=0.014), and the effect of the emergence of COVID-19 on the Tobramycin E. coli resistance was OR = 6.57 (95% Confidence interval (CI) 1.61-7.94). The probability of Tobramycin E.coliresistance linked with poultry meat bought after COVID-19 was 1.88 times more than before COVID-19 emergence. Poultry meat purchased after COVID-19 found related to Tobramycin resistance in E.coli. It seems possible that the overuse of biocides during COVID-19 increased the risk of Tobramycin E.coli resistance in poultry meat.
Key words: Antibiotic; antimicrobial-resistance; biocide; crossresistance; risk-factor
INTRODUCTION
MATERIALS AND METHODS
Sample collection
E.coli isolation and identification
- Brilliant Green Bile Broth with Durham Tube (BGLB): positive gas production,
- Triple Sugar Iron agar (TSIA) : Acid/Acid, gas Positive, H2S negative,
- Citrate: negative,
- Urease: negative,
- Indole: positive.
E.coli antimicrobial susceptibility testing
Statistical analysis
RESULTS AND DISCUSSION
Independent t-test
Chi-squared test (X2 ) and Odds ratio (OR)

Principal component analysis (PCA)
Binary logistic regression
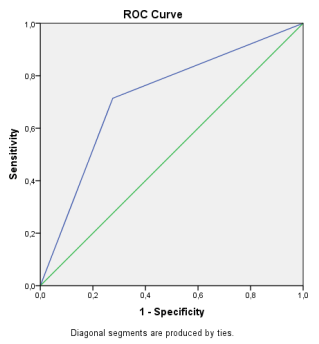
Tobramycin E.coli resistance before COVID-19 appearance
Tobramycin E.coli resistance after COVID-19 appearance
CONCLUSIONS
Conflict of interest
[1] AGATHOKLEOUS, E.; BARCELÓ, D.; IAVICOLI, I.; TSATSAKIS, A.; CALABRESE, E.J. Disinfectant-induced hormesis: An unknown environmental threat of the application of disinfectants to prevent SARS-CoV-2 infection during the COVID-19 pandemic? Environm. Pollution. 292: 118429. 2022.
[2] ALGERIA PRESS SERVICE(APS). 2020. Online: https://bit. ly/3HsTtZi. 10/04/2021.
[3] ANSARI, S.; HAYS, J.P.; KEMP, A.; OKECHUKWU, R.; MURUGAIYAN, J.; EKWANZALA, M.D.; RUIZ-ALVAREZ, M.J.; PAUL-SATYASEELA, M.; DECLAN-IWU, C.; BALLESTE-DELPIERRE, C.; SEPTIMUS, ED.; MUGISHA, L.; FADARE, J.; CHAUDHURI, S.; CHIBABHAI, V.; WADANAMBY, J.M.; DAOUD, Z.; XIAO, Y.; PARKUNAN, T.; KHALAF, Y.; M’IKANATHA, N.; VAN-DONGEN, M.B. The potential impact of the COVID-19 pandemic on global antimicrobial and biocide resistance: an AMR Insights global perspective. JAC Antimicrob. Resist. 3(2): dlab038. 2021.
[4] BAKER-AUSTIN, C.; WRIGHT, M.S.; STEPANAUSKAS, R.; MCARTHUR, J.V. Co-selection of antibiotic and metal resistance. Trends in Microbiol. 14(4): 176–182. 2006.
[5] CHEN, B.; HAN, J.; DAI, H.; JIA, P. Biocide-tolerance and antibioticresistance in community environments and risk of direct transfers to humans: Unintended consequences of community-wide surface disinfecting during COVID-19? Environm. Pollution. 283: 117074. 2021.
[6] DONAGHY, J.A.; JAGADEESAN, B.; GOODBURN, K.; GRUNWALD, L.; JENSEN, O.N.; JESPERS, A.D.; KANAGACHANDRAN, K.; LAFFORGUE, H.; SEEFELDER, W.; QUENTIN, M.C. Relationship of sanitizers, disinfectants, and cleaning agents with antimicrobial resistance. J. Food Prot. 82(5): 889–890. 2019.
[7] DUFORT-ROULEAU, C.; CARIGNAN, A.; BELOIN-JUBINVILLE, B.; LONGPRE, A.A.; DION, J.; LEGELEUX, L.; GILBERT, M. La substitution de la gentamicine par la Tobramycine en contexte de pénurie : évaluation de l’impact sur la résistance bactérienne. Pharmactuel. 53(1): 17–22. 2020.
[8] DUZE, S.T.; MARIMANI, M.; PATEL, M. Tolerance of Listeria monocytogenes to biocides used in food processing environments. Food Microbiol. 97: 103758. 2021.
[9] FOOD AND DRUG ADMINISTRATION (FDA). 2018. BAM Chapter 4: Enumeration of Escherichia coli and the Coliform Bacteria. Bacteriological Analytical Manual (BAM). Food and Drug Administration (FDA). Online: https://bit.ly/3X1KSTf. 01/01/2018.
[10] FORBES, S.; DOBSON, C.B.; HUMPHREYS, G.J.; MCBAIN, A.J. Transient and sustained bacterial adaptation following repeated sublethal exposure to microbicides and a novel human antimicrobial peptide. Antimicrobial Agents Chemotherapy. 58(10): 5809–5817. 2014.
[11] GADEA, R.; FUENTES, M.Á.F.; PULIDO, R.P.; GÁLVEZ, A.; ORTEGA, E. Effects of exposure to quaternary-ammonium-based biocides on antimicrobial susceptibility and tolerance to physical stresses in bacteria from organic foods. Food Microbiol. 63: 58–71. 2017.
[12] GUERGUEB, N.; ALLOUI, N.; AYACHI, A.; AOUN, L.; CHACHOUA, I. Factors Associated with Bacterial Contamination of Poultry Meat at Butcher Shops in Biskra, Algeria. Vet. Stanica. 52(4): 429–437. 2021.
[13] HAMADOUCHE, M.; ALLOUCHE, S. Assessment of preventive measures application against Covid-19 in the workplace. Evaluation de l’application des mesures préventives contre la Covid-19 en milieu de travail. La Tunisie Medicale. 98(08): 625–632. 2020.
[14] INTERNATIONAL BUSINESS MACHINES (IBM). Released 2012. IBM SPSSStatistics for Windows. Version 21.0. Armonk, NY. 2012.
[15] KAMPF, G. Biocidal agents used for disinfection can enhance antibiotic resistance in gram-negative species. Antibiotics. 7(4): 110. 2018.
[16] KOCÚREKOVÁ, T.; KARAHUTOVÁ, L.; BUJŇÁKOVÁ, D. Antimicrobial Susceptibility and Detection of Virulence-Associated Genes in Escherichia coli strains isolated from commercial broilers. Antibiotics. 10(11): 1303. 2021.
[17] MAILLARD, J.Y. Resistance of bacteria to biocides. Microbiol. Spectr. 6(2): 6.2.19 2018.
[18] MARSHALL, B.M.; LEVY, S.B. Food animals and antimicrobials: impacts on human health. Clin. Microbiol. Rev. 24(4): 718–733. 2011.
[19] MINISTRY OF AGRICULTURE AND RURAL DEVELOPMENT OF ALGERIA (MARD). List of medicines for veterinary use registered as of 24/10/2018. 57 pp. 2018.
[20] MORENTE, E.O.; FERNÁNDEZ-FUENTES, M.A.; BURGOS, M.J.G.; ABRIOUEL, H.; PULIDO, R.P.; GALVEZ, A. Biocide tolerance in bacteria. Intern. J. Food Microbiol. 162(1): 3–25. 2013.
[21] NHUNG, N.T.; CHANSIRIPORNCHAI, N.; CARRIQUE-MAS, J.J. Antimicrobial resistance in bacterial poultry pathogens: a review. Front. Vet. Sci. 126(4): 1–17. 2017.
[22] NICHOLS, D.P.; HAPPOLDT, C.L.; BRATCHER, P.E.; CACERES, S.M.; CHMIEL, J.F.; MALCOLM, K.C.; SAAVEDRA, M.T.; SAIMAN, S.; JENNIFER L. TAYLOR-COUSAR, J.L.; NICK, J.A. Impact of azithromycin on the clinical and antimicrobial effectiveness of tobramycin in the treatment of cystic fibrosis. J. Cystic Fibrosis. 16(3): 358–366. 2017.
[23] ALGERIAN DEMOCRATIC AND POPULAR REPUBLIC OFFICIAL JOURNAL. Executive Decree No. 20-159 of June 13, 2020 on the reorganization of home confinement and the measures taken within the framework of the prevention and control system against the spread of Coronavirus (COVID-19), Art 12. N° 35,18. 2020. Online: https://bit.ly/3YmDlj1. 30/10/2020.
[24] O'NEILL, J. Tackling drug-resistant infections globally: final report and recommendations. 2016. Pubkisher Government of the United Kingdom. United Kingdom. Online: https://bit. ly/3X4SaFO. 12/11/2020.
[25] ONICIUC, E.A.; LIKOTRAFITI, E.; ALVAREZ-MOLINA, A.; PRIETO, M.; LÓPEZ, M.; ALVAREZ-ORDÓÑEZ, A. Food processing as a risk factor for antimicrobial resistance spread along the food chain. Curr. Opinion Food Sci. 30: 21–26. 2019.
[26] R CORE TEAM . R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.2021.
[27] RIZVI, S.G.; AHAMMAD, S.Z. COVID-19 and antimicrobial resistance: A cross-study. Sci. Total Environm. 807: 150873. 2022.
[28] SENAPATI, I.A.; MISHRA, R.; KUNDU, A.K.; MISHRA, B.P.; RATH, P.K. Prevalence and Characterization of Escherichia coli from Poultry Meat in Bhubaneswar. Intern. J. Curr. Microbiol. App. Sci. 9(9): 2047–2055. 2020.
[29] SOCIETE FRANÇAISE DE MICROBIOLOGIE. CASFM/EUCAST (Comité de l’Antibiogramme de la Société Françaisede Microbiologie/ European Committee on Antimicrobial Susceptibility Testing guidelines). Recommendations 2019 V.2.0 Mai. 144 pp. 2019.
[30] SOCIETE FRANÇAISE DE MICROBIOLOGIE. 2020. CASFM/ EUCAST (Comité de l’Antibiogramme de la Société Françaisede Microbiologie/European Committee on Antimicrobial Susceptibility Testing guidelines). Recommendations 2020 V.1.1 Avril. 181 pp. 2020.
[31] THOMAS IV, J.C.; OLADEINDE, A.; KIERAN, T.J.; FINGER JR, J.W.; BAYONA‐VÁSQUEZ, N.J.; CARTEE, J.C.; BEASLEY, J.C.; SEAMAN, J.C.; MCARTHUR, J.V.; RHODES JR, O.E.; GLENN, T.C. Co‐occurrence of antibiotic, biocide, and heavy metal resistance genes in bacteria from metal and radionuclide contaminated soils at the Savannah River Site. Microbial. Biotechnol. 13(4): 1179–1200. 2020.
[32] VERRAES, C.; VAN BOXSTAEL, S.; VAN MEERVENNE, E.; VAN COILLIE, E.; BUTAYE, P.; CATRY, B.; DE SCHAETZEN, M.A.; VAN HUFFEL, X.; IMBERECHTS, H.; DIERICK, K.; DAUBE, G.; SAEGERMAN, C.; DE BLOCK, J.; DEWULF, J.; HERMAN, L. Antimicrobial Resistance in the Food Chain: A Review. Intern. J. Environm. Res. Publ. Health. 10(7): 2643–2669. 2013.
[33] WANG, H.H.; SCHAFFNER, D.W. Antibiotic resistance: how much do we know and where do we go from here? Appl. Environm. Microbiol. 77(20): 7093–7095. 2011.
[34] WESGATE, R.; GRASHA, P.; MAILLARD, J.Y. Use of a predictive protocol to measure the antimicrobial resistance risks associated with biocidal product usage. American J. Infect. Contr. 44(4): 458–464. 2016.
[35] WESGATE, R.; MENARD-SZCZEBARA, F.; KHODR, A.; CUPFERMAN, S.; MAILLARD, J.Y. Hydroxyethoxy phenyl butanone, a new cosmetic preservative, does not cause bacterial cross-resistance to antimicrobials. J. Med. Microbiol. 69(5): 670. 2020.
[36] WOOLDRIDGE, M. Evidence for the circulation of antimicrobialresistant strains and genes in nature and especially between humans and animals. Rev. Sci. Tech. 31(1): 231–247. 2012.
[37] ZHANG, L.; KINKELAAR, D.; HUANG, Y.; LI, Y.; LI, X.; WANG, H.H. Acquired antibiotic resistance: are we born with it? Appl. Environm. Microbiol. 77(20): 7134–7141. 2011.









.jpg&w=3840&q=75)