Content sponsored by:
Life Rainbow
Cordyceps militaris hot water extract inhibits lipopolysaccharide-induced inflammatory response in porcine alveolar macrophages by regulation of mitogen-activated protein kinase signaling pathway
Published: July 25, 2017
By: Felix Shih-Hsiang Hsiao 1*, Yeong-Hsiang Cheng 2*, Szu-Kai Wang 2, and Yu-Hsiang Yu 2#
1 Department of Animal Science and Biotechnology, Tunghai University, Taichung, Taiwan
2 Department of Biotechnology and Animal Science, National Ilan University, Yilan, Taiwan
*These authors contributed equally to this work
#Corresponding Author: Yu-Hsiang Yu, Department of Biotechnology and Animal Science, National Ilan University, Yilan City, Yilan County, Taiwan.
ABSTRACT: Cordyceps militaris is a rare and exotic medicinal mushroom used in traditional Chinese medicine. The secondary metabolite, cordycepin (3′-deoxyadenosine), produced from Cordyceps militaris is a biologically active compound. Cordycepin has been demonstrated to exert several pharmacological effects, such as anti-microbial and anti-tumor activities. However, the effect of cordycepin on the immune modulation of porcine alveolar macrophages (PAM) is poorly investigated. In the current study, we investigated the immunomodulatory effect of cordycepin from Cordyceps militaris hot water extract (CMHW) on lipopolysaccharide (LPS)-stimulated PAM. CMHW significantly reduced LPS-stimulated nitric oxide (NO) production and cyclooxygenase-2 (COX-2) protein expression levels in a dose-dependent manner. The LPS-induced phosphorylation of p38 was impaired by CMHW treatment, thereby decreasing pro-inflammatory cytokine secretion, including tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and interleukin-6 (IL-6). Similar to CMHW, cordycepin also had similar effects on LPS-stimulated PAM. Taken together, these findings demonstrate that CMHW has an inhibitory effect on LPS-induced inflammatory responses in PAM through regulation of the p38 mitogen-activated protein kinase (MAPK) signaling pathway. Thus, CMHW is a potential novel feed additive for immunomodulation in farm animals.
Key Words: Cordyceps militaris, Cordycepin; Porcine alveolar macrophage; Lipopolysaccharide; Immunomodulation.
Introduction
Cordyceps militaris is a rare and exotic Chinese medicinal mushroom. Its chemical composition and pharmacological effects are similar to Cordyceps sinensis. Cordyceps sinensis is widely used as a tonic for vitality and longevity for thousands of years by the Chinese (Zhu et al. 1998). Cordyceps militaris has also been used in Chinese medicine for thousands of years. Medicinal fungi have been traditionally used to treat hyposexuality, hyperlipidemia, asthma and lung inflammation (Huang et al. 2004; Guo et al. 2010; Wang et al. 2007). Recently, it has been reported that Cordyceps militaris exerts several pharmacological effects, such as antioxidant, anti-inflammatory, anti-microbial, anti-tumor and anti-angiogenic activities and immunopotentiation (Ng and Wang 2005; Kuo et al. 2007).
The medical potential of the metabolites of Cordyceps militaris has been widely reviewed (Das et al. 2011; Tuli et al. 2013). Cordycepin, or 3'-deoxyadenosine, is the major active secondary metabolite of Cordyceps militaris. The chemical structure of cordycepin is similar to adenosine. It has been reported that the nucleoside analogue exhibits anti-microbial, anti-fungal and anti-tumor activities (Ahn et al. 2000; Sugar and McCaffrey 1998; Nakamura et al. 2006). Cordycepin also has anti-inflammatory effects in LPS-induced murine macrophages and microglia (Kim et al. 2006; Jeong et al. 2010).
Several methodologies have been developed to improve cordycepin productivity, such as liquid culture system or extraction methods (Das et al., 2008; Ni et al., 2006; Das et al., 2010; Wang et al., 2014). However, these methodologies still require high capital investments and operating costs. Alternatively, it has been demonstrated that cordycepin can be produced by solid-state fermentation (Cheng et al. 2016) and subsequent extraction from Cordyceps militaris by water extraction (Kim et al. 2006; Jo et al. 2010). These methodologies are feasible and relatively low cost. However, the extraction procedure and bioactivity of cordycepin still remain to be optimized. In the past decade, studies about the pharmacological and biochemical mechanisms of Cordyceps militaris or cordycepin have all focused on human or mouse models. Recently, we demonstrated that feed supplemented with Cordyceps militaris fermentation products improves growth performance and enhances cell-mediated immunity in piglets (Cheng et al. 2016). PAM are the naturally permissive host cells for respiratory virus infection, such as porcine reproductive and respiratory syndrome virus (PRRSV). But the biochemical mechanism of the immunomodulatory effect of CMHW on PAM has not been elucidated.
The purpose of this study was to investigate the inhibitory effects of cordycepin from CMHW and the mechanism by which it modulates the anti-inflammation mechanism in LPS-stimulated PAM. These results might provide valuable information about the effect of CMHW on immunomodulation by PAM for development as a feed additive.
Materials and Methods
Cordyceps militaris culture
The Cordyceps militaris strain (BCRC 32219) was purchased from the Biosource Collection and Research Center (BCRC, Hsinchu, Taiwan) and was used in solid-state fermentation. Cordyceps militaris was transferred to potato dextrose agar (PDA; Difco Laboratories, Detroit, MI, USA) and incubated at 22°C for 7 days. Cordyceps militaris was then inoculated into an Erlenmeyer flask containing potato dextrose broth (PDB; Difco Laboratories, Detroit, MI, USA) at 22°C for 5 days with shaking.
The Cordyceps militaris strain (BCRC 32219) was purchased from the Biosource Collection and Research Center (BCRC, Hsinchu, Taiwan) and was used in solid-state fermentation. Cordyceps militaris was transferred to potato dextrose agar (PDA; Difco Laboratories, Detroit, MI, USA) and incubated at 22°C for 7 days. Cordyceps militaris was then inoculated into an Erlenmeyer flask containing potato dextrose broth (PDB; Difco Laboratories, Detroit, MI, USA) at 22°C for 5 days with shaking.
Solid-state fermentation of Cordyceps militaris
For solid-state fermentation, the wheat-based substrates containing 0.1% CaCO3, 0.05% MgSO4, 0.1% NaH2PO4, 50% H2O, 0.05% KH2PO4 and 1% glucose were inoculated with a level of 10% (v/w) inoculum, mixed carefully under sterile conditions and were incubated in a chamber with relative humidity above 80%. Cultures were incubated in darkness at 22°C for 2 weeks.
Extraction and analysis of cordycepin
The fermentation product was baked at 50°C for 24 hours and then homogenized by mechanical agitation. The dried fermentation product was heated by distilled deionized water at 100°C for 1 hour and then filtered by a Whatman No. 2 filter. The CMHW filtrate was lyophilized and reconstituted with RPMI-1640 medium (Invitrogen, Carlsbad, CA, USA). The CMHW was then tested for cordycepin content. Cordycepin was quantified according to a previous study with modifications (Chang et al. 2005). In brief, a SPD-10A system (Shimadzu, Columbia, MD, USA) with a programmable UV detector (10A VP, Shimadzu, Columbia, MD, USA) was used in this study. A reverse phase RP-18 column (LiChrospher 100 RP-18 endcapped, 5 µm) protected with a guard was used throughout the experiments. Samples (20 µl) were injected into the HPLC column. The mobile phase consisted of methanol: 0.02 M KH2PO4 (15:85, v/v). The flow rate was 1 ml/min. Cordycepin was determined at a wavelength of 254 nm by use of a UV detector. The recorder was set to 15 min. For cordycepin quantification, linear response was obtained over a range of 100 to 500 µg/ml of the cordycepin standard. Standards were prepared and analyzed a minimum of 3 times. The concentration of cordycepin was determined based on the slope of the standard curve.
Isolation and culture of porcine alveolar macrophages
PAM from 4 week-old male cross-bred (Yorkshire × Landrace) weanling piglets were isolated by Page 5 of 22 Can. J. Anim. Sci. Downloaded from www.nrcresearchpress.com by CORNELL UNIV on 07/18/17 For personal use only. This Just-IN manuscript is the accepted manuscript prior to copy editing and page composition. It may differ from the final official version of record. 6 bronchoalveolar lavages using cold phosphate-buffered saline. Bronchoalveolar fluids were centrifuged at 150 × g for 5 min at 4°C and cell viability was assessed by 0.1% trypan blue exclusion assay. Differential cell counts were performed on cytospin preparations stained with Diff-Quik (Diagnostic Systems, Gibbstown, NJ, USA). The PAM were then frozen in liquid nitrogen in 90% fetal bovine serum (FBS) and 10% dimethyl sulfoxide (DMSO). After thawing, PAM were adjusted to a cell concentration of 2 × 106 cells/mL in growth medium (RPMI-1640 medium containing 10% FBS and 1% penicillin-streptomycin) and cultured 37°C in 5% CO2. PAM were pretreated with the indicated concentrations of the CMHW for 30 min before 1 µg/ml of LPS treatment. After 24 hour of LPS stimulation, the supernatant of culture medium from PAM was harvested and cytokine concentrations were determined. Total protein lysate from LPS-stimulated PAM was extracted and analyzed the protein content by western blot.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay
PAM was seeded into 96-well plates at a density of 5×103 per well and treated with the indicated concentrations of CMHW for 24 hour. For the MTT assay, medium was removed and 20 µl of MTT solution (5 mg/ml in PBS) was added into each well. Cells were incubated at 37°C in 5% CO2 for 4 h. The medium was then removed and 100 µl of DMSO was added into each well. The plate was gently rotated on an orbital shaker for 10 min. The absorbance was detected at 570 nm with a microplate Reader (VersaMax, Molecular Devices, Sunnyvale, CA, USA).
Measurement of NO
NO production was determined by measuring nitrite accumulation. PAM was seeded into 96-well plates at a density of 2×105 per well and pre-treated with the indicated concentrations of CMHW or cordycepin for 1 hour before 1 µg/ml of LPS treatment. After 24 hour of LPS stimulation, 50 µl of supernatant was mixed with 50 µl of Griess reagent (0.1 % naphthylenediamine dihydrochloride, 1 % sulfanilamide in 5% H3PO4, v/v). After 5 min incubation at room temperature, the optical density of the samples was measured by spectrophotometery at 540 nm.
Western blot
Total protein from PAM was extracted by radioimmunoprecipitation assay buffer with protease and phosphatase inhibitors. The sample was centrifuged at 12000 rpm for 10 min and supernatant was subjected to western blot. Protein lysate (20 µg) was separated by sodium dodecyl sulfate–polyacrylamide gel Page 6 of 22 Can. J. Anim. Sci. Downloaded from www.nrcresearchpress.com by CORNELL UNIV on 07/18/17 For personal use only. This Just-IN manuscript is the accepted manuscript prior to copy editing and page composition. It may differ from the final official version of record. 7 electrophoresis and then transblotted onto a polyvinylidine fluoride membrane (Perkin Elmer, Norwalk, CT). The COX-2 primary antibody was purchased from EMD Millipore (Danvers, MA, USA). The phospho-c-Jun N-terminal kinases (p-JNK), phospho-extracellular signal-regulated kinases (p-ERK) and p-p38 primary antibodies were purchased from Sigma-Aldrich (St. Louis, MO, USA). The JNK, ERK and p38 primary antibodies were purchased from Santa Cruz Biotechnology (Dallas, TX, USA). β-actin antibody (EMD Millipore, Danvers, MA, USA) was used for the loading control in the lysates of total protein. The secondary antibody coupled to horseradish peroxidase was used in the chemiluminescence procedure (Immobilon Western, EMD Millipore, Danvers, MA, USA). The Western blotting procedure was performed according to the manufacturer’s instruction.
Cytokine assay
The commercial ELISA kits (R&D Systems, Minneapolis, MN, USA) were used to evaluate the accuracy of the IL-6, TNF-α and IL-1β content. The supernatant from the cultured cells was collected and centrifuged at 1500 rpm for 10 min. The optical density in each kit well was detected using a microplate Reader (VersaMax, Molecular Devices, Sunnyvale, CA, USA) and the cytokine levels which were deduced from the absorbance value by extrapolation from a standard curve generated in parallel.
Ethics statement
All experiments were performed in accordance with the approved guidelines. The animal protocol was approved by the National Ilan University Institutional Animal Care and Use Committee (IACUC Approval No. 104-14).
Statistical analysis
The results were expressed as the percentage subjected to logarithmic transformation prior to analysis of variance. Statistical significance among groups was determined by one-way analysis of variance. Duncan’s new multiple range test was used to evaluate differences between means (SAS Institute, Cary, NC, USA). P values less than 0.05 were considered statistically significant.
Results
Identification of cordycepin from CMHW
To examine the concentration of cordycepin after hot water extraction, CMHW was analyzed by HPLC based on a standard curve. We found that a specific peak was detected with a chromatograph Page 7 of 22 Can. J. Anim. Sci. Downloaded from www.nrcresearchpress.com by CORNELL UNIV on 07/18/17 For personal use only. This Just-IN manuscript is the accepted manuscript prior to copy editing and page composition. It may differ from the final official version of record. 8 identical to standard cordycepin (Fig. 1), indicating that CMHW contains a high amount of cordycepin after hot water extraction. To determine the cytotoxic effect of CMHW, PAM were treated with CMHW at various concentrations for 24 hours. No significant difference was found in cell survival rate even at the highest concentration of 80 µg/ml CMHW (data not shown). These findings demonstrate that hot water extraction is a practically feasible method for cordycepin extraction from solid-state fermentation product of Cordyceps militaris.
Effect of CMHW and cordycepin on inflammatory response in LPS-treated PAM
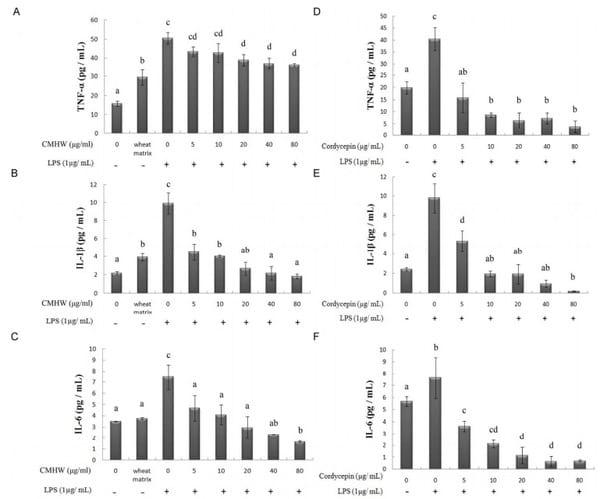
We first investigated whether CMHW could alleviate LPS-induced nitrate biosynthesis in PAM. As expected, LPS significantly promoted nitrate levels in PAM (Fig. 2A and 2B). However, CMHW obviously attenuated LPS-stimulated nitrate production in a dose-dependent manner (Fig. 2A). Cordycepin also consistently had an inhibitory effect on nitrate production (Fig. 2B). We then investigated the effects of CMHW and cordycepin on COX-2 protein expression in LPS-stimulated PAM by western blot analysis. Interestingly, fermentation substrate, the wheat matrix, significantly increased the protein level of COX-2 in PAM (Fig. 3A), indicating that wheat matrix contains potent pro-inflammatory factors. The LPS treatment further promoted COX-2 protein expression in PAM, whereas CMHW significantly alleviated LPS-induced COX-2 protein expression in a concentration-dependent manner at treatments up to 80 µg/ml (Fig. 3A). Similiarly, cordycepin also suppresed COX-2 protein expression in LPS-stimulated PAM (Fig. 3B). Similarly, wheat matrix significantly increased the pro-inflammatory cytokine levels in the culture media, such as TNF-α and IL-1β (Fig. 4A). By contrast, we found no significant effects of wheat matrix on IL-6 levels (Fig. 4A). Furthermore, pro-inflammatory cytokines were increased in the culture media of LPS-stimulated PAM (Fig. 4A-C), while CMHW remarkably attenuated LPS-stimulated pro-inflammatory cytokine production (Fig. 4A-C). Cordycepin also exerted a similar effect on inhibition of pro-inflammatory cytokine levels in LPS-stimulated PAM (Fig. 4D-F). These results demonstrate that CMHW and cordycepin showed a significant inhibitory effect on the production of pro-inflammatory mediators and cytokines in LPS-stimulated PAM.
Effect of CMHW and cordycepin on MAPK signaling pathway in LPS-treated PAM
We next investigated whether CMHW and cordycepin inhibit LPS-induced pro-inflammatory mediators and cytokine production by regulation of the MAPK pathway. Similarly, phosphorylation of Page 8 of 22 Can. J. Anim. Sci. Downloaded from www.nrcresearchpress.com by CORNELL UNIV on 07/18/17 For personal use only. This Just-IN manuscript is the accepted manuscript prior to copy editing and page composition. It may differ from the final official version of record. 9 ERK and p38 were elevated by wheat matrix treatment in PAM (Fig. 5A). As expected, LPS significantly induced the phosphorylation of ERK and p38 in PAM (Fig. 5A). CMHW alleviated the phosphorylation of ERK in LPS-stimulated PAM at low concentration, while this effect was not observed at high concentration of CMHW (Fig. 5A). Interestingly, LPS in combination with CMHW treatment activated the phosphorylation of JNK in PAM compared with LPS treatment alone (Fig. 5A). In addition, CMHW reduced the phosphorylation of p38 in LPS-stimulated PAM in a dose-dependent manner (Fig. 5A). Cordycepin remarkably suppressed the phosphorylation of ERK and p38 in LPS-stimulated PAM in a concentration-dependent manner (Fig. 5B). In contrast, cordycepin attenuated the phosphorylation of JNK in LPS-stimulated PAM in a dose-dependent manner compared with CMHW (Fig. 5B). Taken together, these results indicated that CMHW or cordycepin could inhibit LPS-induced inflammatory mediator production in PAM through suppression of the p38 MAPK signaling pathway.
Discussion
In this study we demonstrated that CMHW could inhibit LPS-stimulated NO production and COX-2 protein expression levels in PAM. The LPS-induced phosphorylation of p38 was attenuated by CMHW, leading to reduced secretion of pro-inflammatory cytokines. The effects of CMHW on immunomodulation in PAM are similar to cordycepin.
Recent studies have demonstrated that cordycepin, the bioactive component isolated from Cordyceps militaris has various pharmacological activities (Ahn et al. 2000; Sugar and McCaffrey 1998; Nakamura et al. 2006). Cordycepin has been shown to have anti-inflammatory activity in LPS-stimulated Raw 264.7 macrophage cells (Kim et al. 2006; Choi et al. 2014; Zhang et al. 2014). Furthermore, it has been demonstrated that cordycepin has an inhibitory effect on pro-inflammatory cytokine expression and secretion in LPS-stimulated murine microglia (Jeong et al. 2010). Similar to previous studies, we also found that cordycepin from CMHW also inhibited pro-inflammatory cytokine secretion in LPS-stimulated PAM. Several possible molecular mechanisms for cordycepin involvement in inhibition of the LPS-induced inflammatory response have been demonstrated. It has been shown that cordycepin suppresses the production of NO by attenuation of inducible NO synthase (iNOS) and COX-2 gene expression via the inhibition of phosphorylation of NF-κB activation, Akt and p38 (Kim et al. 2006; Jeong et al. 2010). The inhibitory effects of cordycepin on LPS-stimulated inflammatory responses in murine macrophages are Page 9 of 22 Can. J. Anim. Sci. Downloaded from www.nrcresearchpress.com by CORNELL UNIV on 07/18/17 For personal use only. This Just-IN manuscript is the accepted manuscript prior to copy editing and page composition. It may differ from the final official version of record. 10 associated with suppression of the MAPK by inhibition of the Toll-like receptor 4 signaling pathway (Choi et al. 2014). Cordycepin inhibits TNF-α production in LPS-stimulated murine macrophage cells through activation of the AMP-activated protein kinase (AMPK)-mediated signalling pathway (Zhang et al. 2014). It has been shown that p38 MAPK plays a key role in LPS-stimulated signal transduction pathways in macrophages (Bode et al. 2012). Here, we also demonstrate that LPS-induced phosphorylation of p38 was impaired in PAM by cordycepin from CMHW, thereby decreasing pro-inflammatory cytokine gene expression. Cumulatively, these findings demonstrate that cordycepin from CMHW alleviates production of pro-inflammatory mediators and cytokines at the transcriptional level by suppression of upstream signaling cascades, such as the MAPK and AMPK signalling pathways.
LPS can induce the production of inflammatory mediators, such as NO and prostaglandin E (Milano et al. 1995). COX-2 is a critical enzyme for prostaglandin E biosynthesis during inflammation (Ricciotti and FitzGerald 2011). Several studies have shown that iNOS and COX2 are highly expressed in macrophages in response to inflammation (Kerwin et al. 1995; Mitchell et al. 1995). NO produced from macrophages by iNOS and COX2 modulation is an important regulatory molecule during inflammation (MacMicking et al. 1997; Coleman 2001). PAM play an important role as the first defense against respiratory infections. It has been reported that inflammatory cytokines, such as TNF-α and IL-1β, are expressed for a short period in LPS-stimulated PAM (Choi et al. 2002; Islam et al. 2013). In the present study, we also found that pro-inflammatory mediators and cytokines were increased in the culture media of LPS-treated PAM. Several functional molecules have been shown to have potent immunomodulatory effects in PAM. It has been demonstrated that β-glucans significantly increase cell activity, phagocytosis and TNF-α production in PAM (Chaung et al. 2009). Wheat β-glucans are linear polysaccharides with repeating glucose units connected by β-1,3 and β-1,4 glycosidic linkages (Havrlentová and Kraic, 2006). These water soluble β-glucans could activate macrophages by dectin-1 and Toll-like receptors, leading to pro-inflammatory mediator and cytokine production such as NO, TNF-α and IL-1β (Abel and Czop, 1992; Brown et al., 2002; Brown et al., 2003; Loures et al., 2014). Consistently, our results demonstrated that wheat matrix containing soluble β-glucans was able to induce NO and cytokine production per se, while CMHW containing cordycepin could reverse the pro-inflammatory effects caused by wheat matrix. However, we found IL-6 levels were not affected by wheat matrix in PAM. These results were consistent with the Page 10 of 22 Can. J. Anim. Sci. Downloaded from www.nrcresearchpress.com by CORNELL UNIV on 07/18/17 For personal use only. This Just-IN manuscript is the accepted manuscript prior to copy editing and page composition. It may differ from the final official version of record. 11 previous reports that IL-6 production was not regulated in broilers in response to β-glucans (Guo et al., 2003). The explanation of the difference may be due to T-lymphocyte activation (Jacob and Pescatore, 2014). TNF-α and IL-1β are mainly produced from type 1 helper T-lymphocytes (Th1) in response to IL-12 stimulation and these cytokines are involved in the cell-mediated immunity. By contrast, IL-6 is mainly produced from type 2 helper T-lymphocytes (Th2) under IL-4 stimulation and IL-6 is involved in the humoral immune response. Thus, wheat β-glucans may primarily induce TNF-α and IL-1β production in PAM by activation of Th1. Furthermore, we found that CMHW significantly reduced the IL-6 production in the LPS-stimulated PAM in a concentration-dependent manner. These results were consistent with the previous reports (Jo et al. 2010). Additionally, we also demonstrated that the inhibitory effect of cordycepin on pro-inflammatory was greater than CMHW in PAM. Recently, our previous study showed that feed supplementation with Cordyceps militaris fermentation products improve growth performance and enhance cell-mediated immunity in piglets (Cheng et al. 2016). Here, we also found that CMHW has an inhibitory effect on LPS-induced inflammatory responses in PAM. These results indicate that modulation of PAM activity by CMHW serves an effective target for immunomodulation in animals.
Water extraction is commonly considered to be a reliable extraction technique that could efficiently harvest potent bioactive molecules. This method is also widely used for isolation and characterization of bioactive compounds from plants. It has been demonstrated that water extract of Cordyceps militaris promotes maturation of murine bone marrow-derived dendritic cells (Kim et al. 2006). Hot water extract from Cordyceps militaris fruiting bodies exerts an inhibitory effect on NO production, TNF-α and IL-6 secretion in LPS-stimulated murine macrophage cells (Jo et al. 2010). Consistently, we also demonstrated that CMHW attenuated pro-inflammatory cytokine secretion in LPS-stimulated PAM, including TNF-α, IL-1β and IL-6. Overall, these findings suggest that water extraction is a feasible method for isolation of bioactive compound from Cordyceps militaris.
In conclusion, in this study we provide evidence that CMHW has an inhibitory effect on LPS-induced inflammatory responses in PAM through regulation of the p38 MAPK signaling pathway. Cordycepin produced in solid-state fermentation and extracted by hot water has high potential for development as a feed additive by providing an alternative source for immunomodulation in farm animals.
Acknowledgements
This work was supported by the Council of Agriculture [96 Agri-2.1.3-Mu-U1(3)] in Taiwan.
References
1. Ahn, Y.J., Park, S.J., Lee, S.G., Shin, S.C., and Choi, D. H. 2000. Cordycepin: selective growth inhibitor derived from liquid culture of Cordyceps militaris against Clostridium spp. J. Agri. Food Chem. 48(7): 2744-2748.
2. Bode, J.G., Ehlting, C., and Häussinger, D. 2012. The macrophage response towards LPS and its control through the p38(MAPK)-STAT3 axis. Cell. Signal. 24(6):1185-1194.
3. Brown, G.D., Taylor, P.R., Reid, D.M., Willment, J.A., Williams, D.L., Martinez-Pomares, L., Wong, S.Y., and Gordon, S. 2002. Dectin-1 is a major beta-glucan receptor on macrophages. J. Exp. Med. 196(3):407-412.
4. Brown, G.D., Herre, J., Williams, D.L., Willment, J.A., Marshall, A.S., and Gordon, S. 2003. Dectin-1 mediates the biological effects of beta-glucans. J. Exp. Med. 197(9):1119-1124.
5. Cheng, Y.H., Wen, C.M., Dybus, A., and Proskura, W.S. 2016. Fermentation products of Cordyceps militaris enhance performance and modulate immune response of weaned piglets. S. Afr. J. Anim. Sci. 46(2):121-128.
6. Chang, C.Y., Lue M.Y., and Pan, T.M. 2005 Determination of adenosine, cordycepin and ergosterol contents in cultivated antrodia camphorate by HPLC method. J. Food Drug. Ana. 13:338-342.
7. Chaung, H.C., Huang, T.C., Yu, J.H., Wu, M.L., and Chung, W.B. 2009. Immunomodulatory effects of beta-glucans on porcine alveolar macrophages and bone marrow haematopoietic cell-derived dendritic cells. Vet. Immunol. Immunopathol. 131(3-4):147-157.
8. Choi, I.S., Shin, N.R., Shin, S.J., Lee, D.Y., Cho Y.W., and Yoo, H.S. 2002. Time course study of cytokine mRNA expression in LPS-stimulated porcine alveolar macrophages. J. Vet. Sci. 3(2):97-102.
9. Coleman, J.W. 2001. Nitric oxide in immunity and inflammation. Int. Immunopharmacol. 1(8):1397-1406.
10. Choi, Y.H., Kim, G.Y., and Lee, H.H. 2014. Anti-inflammatory effects of cordycepin in lipopolysaccharide-stimulated RAW 264.7 macrophages through Toll-like receptor 4-mediated suppression of mitogen-activated protein kinases and NF-κB signaling pathways. Drug Des. Devel. Ther. 16(8):1941-1953.
11. Das, S.K., Masuda, M., Hatashita, M., Sakurai, A., and Sakakibara, M. 2008. A new approach for improving cordycepin productivity in surface liquid culture of Cordyceps militaris using high-energy ion beam irradiation. Lett. Appl. Microbiol. 47(6):534-538.
12. Das, S.K., Masuda, M., Sakurai, A., and Sakakibara, M. 2010. Medicinal uses of the mushroom Cordyceps militaris: current state and prospects. Fitoterapia 81(8):961-968.
13. Das, S.K., Masuda, M., Hatashita, M., Sakurai, A., and Sakakibara, M. 2010. Optimization of culture medium for cordycepin production using Cordyceps militaris mutant obtained by ion beam irradiation. Process Biochem. 45(1):129-132.
14. Guo, Y., Ali, R.A., and Qureshi, M.A. 2003. The influence of beta-glucan on immune responses in broiler chicks. Immunopharmacol. Immunotoxicol. 25(3):461-472.
15. Guo, P., Kai, Q., Gao, J., Lian, Z.Q., Wu, C.M., Wu, C.A., and Zhu, H.B. 2010. Cordycepin prevents hyperlipidemia in hamsters fed a high-fat diet via activation of AMP-activated protein kinase. J. Pharmacol. Sci. 113(4):395-403.
16. Havrlentová, M., and Kraic, J. 2006. Content of β-D-glucan in cereal grains. J. Food Nutr. Res. 45(3):97-103.
17. Huang, Y.L., Leu, S.F., Liu, B.C., Sheu, C.C., and Huang, B.M. 2004. In vivo stimulatory effect of Cordyceps sinensis mycelium and its fractions on reproductive functions in male mouse. Life Sci. 75(9):1051-1062.
18. Islam, M.A., Pröll, M., Hölker, M., Tholen, E., Tesfaye, D., Looft, C., Schellander, K., and Cinar, M. U. 2013. Alveolar macrophage phagocytic activity is enhanced with LPS priming, and combined stimulation of LPS and lipoteichoic acid synergistically induce pro-inflammatory cytokines in pigs. Innate Immun. 19(6):631-643.
19. Jacob, J.P., and Pescatore, A.J. 2014. Barley β-glucan in poultry diets. Ann. Transl. Med. 2(2):20.
20. Jo, W.S., Choi, Y.J., Kim, H.J., Lee, J.Y., Nam, B.H., Lee, J.D., Lee, S.W., Seo, S.Y., and Jeong, M.H. 2010. The anti-inflammatory effects of water extract from Cordyceps militaris in murine macrophage. Mycobiology 38(1):46-51.
21. Jeong, J.W., Jin, C.Y., Kim, G.Y., Lee, J.D., Park, C., Kim, G.D., Kim, W.J., Jung, W.K., Seo, S.K., Choi, I.W., and Choi, Y.H. 2010. Anti-inflammatory effects of cordycepin via suppression of Page 14 of 22 Can. J. Anim. Sci. Downloaded from www.nrcresearchpress.com by CORNELL UNIV on 07/18/17 For personal use only. This Just-IN manuscript is the accepted manuscript prior to copy editing and page composition. It may differ from the final official version of record. 15 inflammatory mediators in BV2 microglial cells. Int. Immunopharmacol. 10(12):1580-1586.
22. Kuo, M.C., Chang, C.Y., Cheng, T.L., and Wu, M.J. 2007. Immunomodulatory effect of exo-polysaccharides from submerged cultured Cordyceps sinensis: enhancement of cytokine synthesis, CD11b expression, and phagocytosis. Appl. Microbiol. Biotechnol. 75(4):769-775.
23. Kim, H.G., Shrestha, B., Lim, S.Y., Yoon, D.H., Chang, W.C., Shin, D.J., Han, S.K., Park, S.M., Park, J.H., Park, H.I., Sung, J.M., Jang, Y., Chung, N., Hwang, K.C., and Kim, T.W. 2006. Cordycepin inhibits lipopolysaccharide-induced inflammation by the suppression of NF-κB through Akt and p38 inhibition in RAW 264.7 macrophage cells. Eur. J. Pharmacol. 545(2-3):192-199.
24. Kim, G.Y., Ko, W.S., Lee, J.Y., Lee, J.O., Ryu, C.H., Choi, B.T., Park, Y.M., Jeong, Y.K., Lee, K.J., Choi, K.S., Heo, M.S., and Choi, Y.H. 2006. Water extract of Cordyceps militaris enhances maturation of murine bone marrow-derived dendritic cells in vitro. Biol. Pharm. Bull. 29(2):354-360.
25. Kerwin, J.F.Jr., Lancaster, J.R.Jr., and Feldman, P. L. 1995. Nitric oxide: a new paradigm for second messengers. J. Med. Chem. 38(22):4343-4362.
26. Loures, F.V., Araújo, E.F., Feriotti, C., Bazan, S.B., Costa, T.A., Brown, G.D., and Calich, V.L. 2014. Dectin-1 induces M1 macrophages and prominent expansion of CD8+IL-17+ cells in pulmonary Paracoccidioidomycosis. J. Infect. Dis. 210(5):762-773.
27. MacMicking, J., Xie, Q.W., and Nathan, C. 1997. Nitric oxide and macrophage function. Ann. Rev. Immunol. 15:323-330.
28. Milano, S., Arcoleo, F., Dieli, M., D'Agostino, R., D'Agostino, P., De Nucci, G., and Cillari, E. 1995. Prostaglandin E2 regulates inducible nitric oxide synthase in the murine macrophage cell line J774. Prostaglandins 49(2):105-115.
29. Mitchell, J.A., Larkin, S., and Williams, T.J. 1995. Cyclooxygenase-2: regulation and relevance in inflammation. Biochem. Pharmacol. 50(10):1535-1542.
30. Ng, T.B., and Wang, H.X. 2005. Pharmacological actions of Cordyceps, a prized folk medicine. J. Pharmacol. 57(12):1509-1519.
31. Nakamura, K., Yoshikawa, N., Yamaguchi, Y., Kagota, S., Shinozuka, K., and Kunitomo, M. 2006. Antitumor effect of cordycepin (3'-deoxyadenosine) on mouse melanoma and lung carcinoma cells involves adenosine A3 receptor stimulation. Anticancer Res. 26(1A):43-47.
32. Ni, H., Zhou, X.H., Li, H.H., and Huang, W.F. 2009. Column chromatographic extraction and preparation of cordycepin from Cordyceps militaris waster medium. J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 877(22):2135-2141.
33. Ricciotti, E., and FitzGerald, G.A. 2011. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 31(5):986-1000.
34. Sugar, A.M., and McCaffrey, R.P. 1998. Antifungal activity of 3‘-deoxyadenosine (cordycepin). Antimicrob. Agents Chemother. 42(6):1424-1427.
35. Tuli, H.S., Sharma, A.K., Sandhu, S.S., and Kashyap, D. 2013. Cordycepin:a bioactive metabolite with therapeutic potential. Life Sci. 93(23):863-869.
36. Wang, N.Q., Jiang, L.D., Zhang, X.M., and Li, Z.X. 2007. Effect of dongchong xiacao capsule on airway inflammation of asthmatic patients. Zhongguo Zhong Yao Za Zhi 32(15):1566-1568.
37. Wang, H.J., Pan, M.C., Chang, C.K., Chang, S.W., and Hsieh, C.W. 2014. Optimization of ultrasonic-assisted extraction of cordycepin from Cordyceps militaris using orthogonal experimental design. Molecules 19(12):20808-20820.
38. Zhang, J.L., Xu, Y., and Shen, J. 2014. Cordycepin inhibits lipopolysaccharide (LPS)-induced tumor necrosis factor (TNF)-α production via activating amp-activated protein kinase (AMPK) signaling. Int. J. Mol. Sci. 15(7):12119-12134.
39. Zhu, J.S., Halpern, G.M., and Jones, K. 1998. The scientific rediscovery of an ancient Chinese herbal medicine: Cordyceps sinensis: part I. J. Altern. Complement. Med. 4(3):289-303.
Figure legends
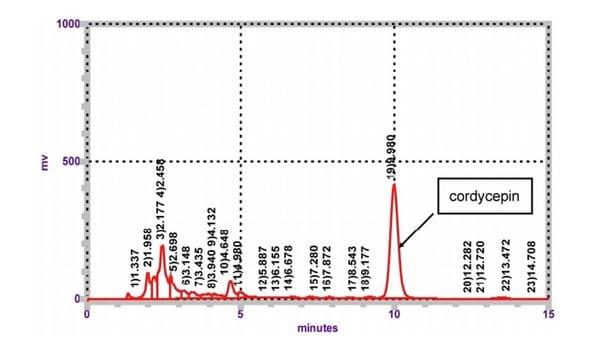
Figure 1. Identification of cordycepin from CMHW. Measurement of CMHW by HPLC. Three experiments were carried out, and one representative result is shown.
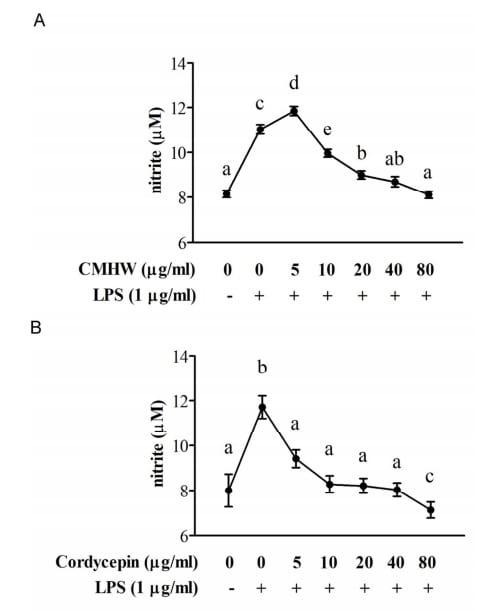
Figure 2. Effect of CMHW and cordycepin on NO levels in LPS-stimulated PAM. PAM was pre-treated with (A) various concentrations of CMHW (0-80 µg/ml) and (B) cordycepin (0-80 µg/ml) for 1 hour and then stimulated with 1 µg/ml LPS for 24 hours. The NO from supernatant of culture medium was analyzed using Griess reagent. Values were expressed as mean ± SEM (n=6). a-eMeans in the same superscript followed by different letters are significantly different (P < 0.05).
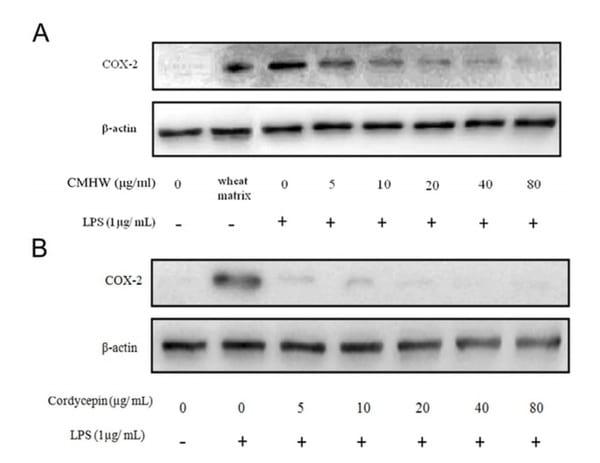
Figure 3. Effect of CMHW and cordycepin on LPS-induced COX-2 protein expression in PAM. PAM were pre-treated with (A) various concentrations of CMHW (0-80 µg/ml) and (B) cordycepin (0-80 µg/ml) for 1 hour and then stimulated with 1 µg/ml LPS for 24 hours. The protein levels of COX-2 and β-actin were determined by immunoblotting analysis. Three experiments were carried out, and one representative result is shown.
Figure 4. Effect of CMHW and cordycepin on LPS-induced TNF-α (A and D), IL-1β (B and E) and IL-6 (C and F) in PAM. PAM were pre-treated with various concentrations of CMHW (0-80 µg/ml) and cordycepin (0-80 µg/ml) for 1 hour and then stimulated with 1 µg/ml LPS for 24 hours. The pro-inflammatory cytokines levels in culture medium were assayed by ELISA. Values were expressed as mean ± SEM (n=3). a-dMeans in the same superscript followed by different letters are significantly different (P < 0.05).
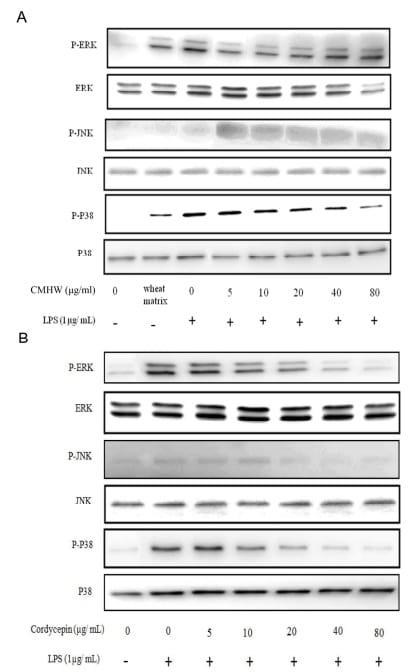
Figure 5. Effect of CMHW and cordycepin on LPS-induced phosphorylation of MAPK pathway in PAM. PAM were pre-treated with (A) various concentrations of CMHW (0-80 µg/ml) and (B) cordycepin (0-80 µg/ml) for 1 hour and then stimulated with 1 µg/ml LPS for 24 hours. The phosphor- or total protein of MAPK (ERK, JNK and p38) from cell lysates were determined by phosphor-specific or specific protein antibodies. Three experiments were carried out, and one representative result is shown.
Related topics:
Authors:

Recommend
Comment
Share

Would you like to discuss another topic? Create a new post to engage with experts in the community.