Spray Dried Porcine Blood Products Are Safe Porcine Epidemic Diarrhea Virus
Published: February 18, 2014
Source : APC
The North American Spray Dried Blood and Plasma Producers (NASDBPP; Table 1) are committed to producing safe, high-quality blood products for use in feeds for commercial livestock and companion animals. Recent publicity concerning the transmission of Porcine Epidemic Diarrhea virus (PEDv) has resulted in questions about the potential role of spray dried porcine plasma and porcine red cells in the spread of this disease.
Based on current scientific evidence, the NASDBPP concludes that properly sourced, collected and processed porcine blood and porcine blood products are safe and do not contribute to the spread of PEDv. In 1994, the NASDBPP member companies developed Good Manufacturing Practices (GMPs) to assure the proper sourcing, collecting and processing of animal blood and blood products to maintain safety. These GMPs provide multiple safeguards for the safety and quality of spray-dried blood and plasma products. The members actively support continued research into the causes and control of this and other diseases.
Current scientific evidence indicates collection and manufacturing processes ensure spray dried bovine or porcine blood and bovine or porcine plasma products are safe feed ingredients.
Based on current scientific evidence, the NASDBPP concludes that properly sourced, collected and processed porcine blood and porcine blood products are safe and do not contribute to the spread of PEDv. In 1994, the NASDBPP member companies developed Good Manufacturing Practices (GMPs) to assure the proper sourcing, collecting and processing of animal blood and blood products to maintain safety. These GMPs provide multiple safeguards for the safety and quality of spray-dried blood and plasma products. The members actively support continued research into the causes and control of this and other diseases.
Current scientific evidence indicates collection and manufacturing processes ensure spray dried bovine or porcine blood and bovine or porcine plasma products are safe feed ingredients.
Characteristics of PED
- Porcine Epidemic Diarrhea virus is classified in the Coronaviridae family.
- Clinically, PEDv infection is similar to transmissible gastroenteritis (TGE), which is also a Coronavirus.
- PEDv is an RNA enveloped virus ranging in diameter from 90 to 190 nm.
- PEDv is stable at low temperatures in neutral pH.
o 39.0oF (4.0oC) at pH between 5.0 and 9.0
o 98.5oF (37oC) at pH between 6.5 and 7.5 - High temperatures inactivate PEDv
o140oF (60oC) for 30 min
o 160oF (71oC) for 10 min - PEDv is inactivated by most virucidal disinfectants (Pospischil et al., 2002)
o sodium hydroxide (2%),
o formalin (1%),
o sodium carbonate (4%),
o ionic and non-ionic detergents
o lipid solvents such as chloroform
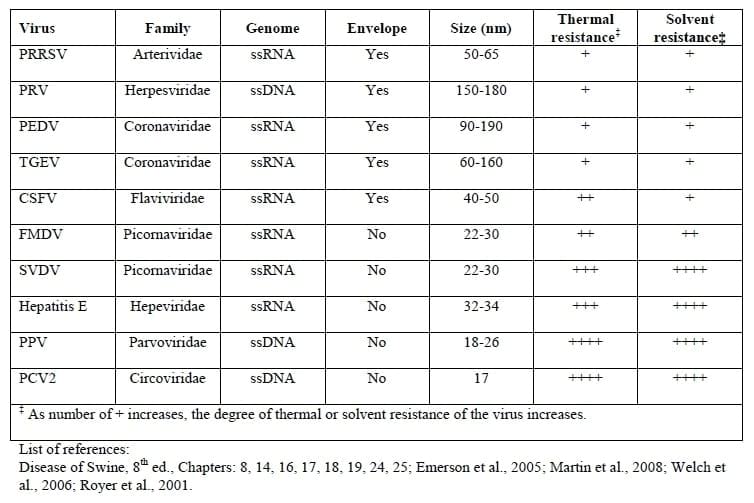
Spray Drying has been shown to inactivate many viruses (Table 2).
The NASDBPP has supported research documenting that spray drying inactivates many different viruses. Porcine Reproductive and Respiratory Syndrome virus (PRRSv) is an envelope virus with stability characteristics very similar to PEDv. Spray drying has been shown to inactivate PRRSv (Polo et al., 2005). In addition, spray drying has been shown to inactivate Pseudorabies virus (Polo et al., 2005) and Swine Vesicular Disease virus (Pujols et al., 2007). Spray dried blood products are heated to over 175oF (80oC) throughout substance - well above temperatures shown to inactivate PEDv.
The NASDBPP has supported research documenting that spray drying inactivates many different viruses. Porcine Reproductive and Respiratory Syndrome virus (PRRSv) is an envelope virus with stability characteristics very similar to PEDv. Spray drying has been shown to inactivate PRRSv (Polo et al., 2005). In addition, spray drying has been shown to inactivate Pseudorabies virus (Polo et al., 2005) and Swine Vesicular Disease virus (Pujols et al., 2007). Spray dried blood products are heated to over 175oF (80oC) throughout substance - well above temperatures shown to inactivate PEDv.
Spray drying is recognized by international government agencies as an effective heat treatment to inactivate PEDv.
Department for Environmental, Food and Rural Affairs (DEFRA) International Disease Monitoring (IDM) team stated that spray drying and heat treatment used in the manufacture of blood products were effective to inactivate PEDv (VITT/1200 PED in USA; 24/07/2013).
Many viruses do not survive in a dry environment
Recent data has demonstrated that when inoculated in dry feed, PEDv did not survive past 7 days when held at room temperature. In contrast, in a liquid slurry (5 g feed mixed with 10 ml water), PEDv survived 28 days. Spray dried blood products have a moisture content of 8% and a water activity < 0.60. Typically, spray dried blood products will be held for a minimum of 7 – 10 days pending QA analysis with an additional 2 weeks to move through commercial channels. Depending on inventory levels and demand, typical product age will range from 1 month to 2 years. Envelope viruses, like PEDv, do not survive in dry environments; therefore, PEDv would not be expected to survive in spray dried blood products.
The presence of viral genome in spray dried blood product does not mean that feeding the ingredient will transmit the disease.
It is common for commercial porcine plasma to test positive for the presence of Porcine Circovirus (PCV2) DNA. However, pigs did not become infected with PCV2 when fed a diet
Department for Environmental, Food and Rural Affairs (DEFRA) International Disease Monitoring (IDM) team stated that spray drying and heat treatment used in the manufacture of blood products were effective to inactivate PEDv (VITT/1200 PED in USA; 24/07/2013).
Many viruses do not survive in a dry environment
Recent data has demonstrated that when inoculated in dry feed, PEDv did not survive past 7 days when held at room temperature. In contrast, in a liquid slurry (5 g feed mixed with 10 ml water), PEDv survived 28 days. Spray dried blood products have a moisture content of 8% and a water activity < 0.60. Typically, spray dried blood products will be held for a minimum of 7 – 10 days pending QA analysis with an additional 2 weeks to move through commercial channels. Depending on inventory levels and demand, typical product age will range from 1 month to 2 years. Envelope viruses, like PEDv, do not survive in dry environments; therefore, PEDv would not be expected to survive in spray dried blood products.
The presence of viral genome in spray dried blood product does not mean that feeding the ingredient will transmit the disease.
It is common for commercial porcine plasma to test positive for the presence of Porcine Circovirus (PCV2) DNA. However, pigs did not become infected with PCV2 when fed a diet
containing high levels of porcine plasma with high titers for PCV2 DNA (Pujols et al., 2008; Shen et al., 2011; Polo et al., 2013). Rovira, 2013, released data demonstrating that pigs did not become infected when fed a diet PCR positive for PEDv. PCR testing does not reflect infectivity.
Manufacturing standards:
The NASDBPP has developed manufacturing standards ensuring that spray dried blood products are collected in a hygienic manner and processed in such a way to prevent contamination.
Manufacturing standards:
The NASDBPP has developed manufacturing standards ensuring that spray dried blood products are collected in a hygienic manner and processed in such a way to prevent contamination.
- Blood is collected only from healthy animals fit for slaughter for human consumption in federally inspected slaughter houses.
- During collection blood is immediately pumped through a closed system to a processing room isolated from the slaughter area.
- Blood is transported in dedicated tankers to a remote spray drying facility.
- Packaging material is new.
In summary, envelope viruses, like PEDv, are inactivated by heat treatment and do not survive in dry environments. Spray drying has been shown to inactivate many viruses including envelope viruses. International Scientific Review Committees recognize spray drying inactivates PEDv. PCR testing for PEDv does not determine if a feed ingredient is infective. The NASDBPP has developed manufacturing standards insuring that spray dried blood products are collected in a hygienic manner and processed in such a way to prevent contamination. Spray dried porcine plasma and spray dried porcine blood cells are safe, high-quality feed ingredients for livestock and companion animal feed.
References:
Benfield DA, Collins JE, Dee SA, Halbur PG, Joo HS, Lager KM, Mengeling WL, Murtaugh MP, Rossow KD, Stevenson GW, Zimmerman JJ. Porcine Reproductive and Respiratory Syndrome. In: Straw BE, D’Allaire S, Mengeling WL, Taylor DJ, eds. Diseases of Swine, 8th ed. Ames: Iowa State University Press; 1999:201-232.
DEFRA - Department for Environment, Food and Rural Affairs Veterinary & Science Policy Advice International Disease Monitoring. Reference: VITT/1200 PED in USA Date: 24/07/2013
Emerson SU, Arankalle VA, Purcell RH. Thermal stability of hepatitis E virus. J. Infect. Dis. 2005;192:930-933.
House JA, House CA. Vesicular Diseases. In: Straw BE, D’Allaire S, Mengeling WL, Taylor DJ, eds. Diseases of Swine, 8th ed. Ames: Iowa State University Press; 1999:327-340.
Kluge JP, Beran GW, Hill HT, Platt KB. Pseudorabies (Aujeszky’s Disease). In: Straw BE, D’Allaire S, Mengeling WL, Taylor DJ, eds. Diseases of Swine, 8th ed. Ames: Iowa State University Press; 1999:233-246.
Lukert PD, Allan GM. Porcine Circovirus. In: Straw BE, D’Allaire S, Mengeling WL, Taylor DJ, eds. Diseases of Swine, 8th ed. Ames: Iowa State University Press; 1999:119-124.
Martin H, Le Potier MF, Maris P. Virucidal efficacy of nine commercial disinfectants against porcine circovirus type 2. Vet J. 2008;177:388-393.
Mengeling WL. Porcine Parvovirus. In: Straw BE, D’Allaire S, Mengeling WL, Taylor DJ, eds. Diseases of Swine, 8th ed. Ames: Iowa State University Press; 1999:187-200.
National Pork Board PED Research Update. Environmental Stability of PEDV. Project 2. Update 11-13-13.
Pensaert MB. Porcine Epidemic Diarrhea. In: Straw BE, D’Allaire S, Mengeling WL, Taylor DJ, eds. Diseases of Swine, 8th ed. Ames: Iowa State University Press; 1999:179-186.
Polo, J., J. D. Quigley, L. E. Russell, J. M. Campbell, J. Pujols, and P. D. Lukert. 2005. Efficacy of spray-drying to reduce infectivity of Pseudorabies and PRRS viruses and seroconversion in pigs fed diets containing spray-dried animal plasma. J. Anim. Sci. 83:1933-1938.
Polo, J., T. Opriessnig, K.C. O’Neill, C. Rodríguez, L.E. Russell, J.M. Campbell, J. Crenshaw, J. Segalés and J. Pujols. 2013. Neutralizing antibodies against porcine circovirus type 2 in liquid pooled plasma contribute to the biosafety of commercially manufactured spray-dried porcine plasma. J. Anim. Sci. 91:2192-2198.
Pospischil, A., A. Stuedli, and M. Kiupel, 2002. Update on porcine epidemic diarrhea. J Swine Health Prod. 10(2):81–85.
Pujols, J. R. Rosell, L. Russell, J. Campbell, J. Crenshaw, E. Weaver, C. Rodriguez, J. Rodenas, and J. Polo. 2007. Inactivation of swine vesicular disease virus in porcine plasma by spray-drying. Proc. Amer. Assoc. Swine Vet., p 281-283.
Benfield DA, Collins JE, Dee SA, Halbur PG, Joo HS, Lager KM, Mengeling WL, Murtaugh MP, Rossow KD, Stevenson GW, Zimmerman JJ. Porcine Reproductive and Respiratory Syndrome. In: Straw BE, D’Allaire S, Mengeling WL, Taylor DJ, eds. Diseases of Swine, 8th ed. Ames: Iowa State University Press; 1999:201-232.
DEFRA - Department for Environment, Food and Rural Affairs Veterinary & Science Policy Advice International Disease Monitoring. Reference: VITT/1200 PED in USA Date: 24/07/2013
Emerson SU, Arankalle VA, Purcell RH. Thermal stability of hepatitis E virus. J. Infect. Dis. 2005;192:930-933.
House JA, House CA. Vesicular Diseases. In: Straw BE, D’Allaire S, Mengeling WL, Taylor DJ, eds. Diseases of Swine, 8th ed. Ames: Iowa State University Press; 1999:327-340.
Kluge JP, Beran GW, Hill HT, Platt KB. Pseudorabies (Aujeszky’s Disease). In: Straw BE, D’Allaire S, Mengeling WL, Taylor DJ, eds. Diseases of Swine, 8th ed. Ames: Iowa State University Press; 1999:233-246.
Lukert PD, Allan GM. Porcine Circovirus. In: Straw BE, D’Allaire S, Mengeling WL, Taylor DJ, eds. Diseases of Swine, 8th ed. Ames: Iowa State University Press; 1999:119-124.
Martin H, Le Potier MF, Maris P. Virucidal efficacy of nine commercial disinfectants against porcine circovirus type 2. Vet J. 2008;177:388-393.
Mengeling WL. Porcine Parvovirus. In: Straw BE, D’Allaire S, Mengeling WL, Taylor DJ, eds. Diseases of Swine, 8th ed. Ames: Iowa State University Press; 1999:187-200.
National Pork Board PED Research Update. Environmental Stability of PEDV. Project 2. Update 11-13-13.
Pensaert MB. Porcine Epidemic Diarrhea. In: Straw BE, D’Allaire S, Mengeling WL, Taylor DJ, eds. Diseases of Swine, 8th ed. Ames: Iowa State University Press; 1999:179-186.
Polo, J., J. D. Quigley, L. E. Russell, J. M. Campbell, J. Pujols, and P. D. Lukert. 2005. Efficacy of spray-drying to reduce infectivity of Pseudorabies and PRRS viruses and seroconversion in pigs fed diets containing spray-dried animal plasma. J. Anim. Sci. 83:1933-1938.
Polo, J., T. Opriessnig, K.C. O’Neill, C. Rodríguez, L.E. Russell, J.M. Campbell, J. Crenshaw, J. Segalés and J. Pujols. 2013. Neutralizing antibodies against porcine circovirus type 2 in liquid pooled plasma contribute to the biosafety of commercially manufactured spray-dried porcine plasma. J. Anim. Sci. 91:2192-2198.
Pospischil, A., A. Stuedli, and M. Kiupel, 2002. Update on porcine epidemic diarrhea. J Swine Health Prod. 10(2):81–85.
Pujols, J. R. Rosell, L. Russell, J. Campbell, J. Crenshaw, E. Weaver, C. Rodriguez, J. Rodenas, and J. Polo. 2007. Inactivation of swine vesicular disease virus in porcine plasma by spray-drying. Proc. Amer. Assoc. Swine Vet., p 281-283.
Pujols, J., S. Lopez-Soria, J. Segalés, M. Fort, M. Sibila, R. Rosell, D. Solanes, L. Russell, J. Campbell, J. Crenshaw, E. Weaver, and J. Polo. 2008. Lack of transmission of porcine circovirus type 2 to weanling pigs by feeding them spray-dried porcine plasma. Vet. Rec. 163:536-538.
Royer RL, Nawagigul P, Halbur PG, Paul PS. Susceptibility of porcine circovirus type 2 to commercial and laboratory disinfectants. J Swine Health Prod. 2001;9:281-284.
Rovira, A. 2013. Porcine Epidemic Diarrhea Virus Bioassay. Center for Veterinary Medicine. University of Minnesota.
Saif LJ, Wesley RD. Transmissible Gastroenteritis and Porcine Respiratory Coronavirus. In: Straw BE, D’Allaire S, Mengeling WL, Taylor DJ, eds. Diseases of Swine, 8th ed. Ames: Iowa State University Press; 1999:295-326.
Shen, H.G., S. Schalk, P.G. Halbur, J.M. Campbell, L.E. Russell and T. Opriessnig. 2011. Commercially produced spray-dried porcine plasma contains high levels of porcine circovirus type 2 (PCV2) DNA but did not transmit PCV2 when fed to naïve pigs. J. Anim. Sci. 89:1930-1938.
Van Oirschot JT. Classical Swine Fever (Hog Cholera). In: Straw BE, D’Allaire S, Mengeling WL, Taylor DJ, eds. Diseases of Swine, 8th ed. Ames: Iowa State University Press; 1999:159-172.
Welch J, Bieneck C, Gomperts E, Simmonds P. Resistance of porcine circovirus and chicken anemia virus to virus inactivation procedures used for blood products. Transfusion. 2006;46:1951-1958.
Royer RL, Nawagigul P, Halbur PG, Paul PS. Susceptibility of porcine circovirus type 2 to commercial and laboratory disinfectants. J Swine Health Prod. 2001;9:281-284.
Rovira, A. 2013. Porcine Epidemic Diarrhea Virus Bioassay. Center for Veterinary Medicine. University of Minnesota.
Saif LJ, Wesley RD. Transmissible Gastroenteritis and Porcine Respiratory Coronavirus. In: Straw BE, D’Allaire S, Mengeling WL, Taylor DJ, eds. Diseases of Swine, 8th ed. Ames: Iowa State University Press; 1999:295-326.
Shen, H.G., S. Schalk, P.G. Halbur, J.M. Campbell, L.E. Russell and T. Opriessnig. 2011. Commercially produced spray-dried porcine plasma contains high levels of porcine circovirus type 2 (PCV2) DNA but did not transmit PCV2 when fed to naïve pigs. J. Anim. Sci. 89:1930-1938.
Van Oirschot JT. Classical Swine Fever (Hog Cholera). In: Straw BE, D’Allaire S, Mengeling WL, Taylor DJ, eds. Diseases of Swine, 8th ed. Ames: Iowa State University Press; 1999:159-172.
Welch J, Bieneck C, Gomperts E, Simmonds P. Resistance of porcine circovirus and chicken anemia virus to virus inactivation procedures used for blood products. Transfusion. 2006;46:1951-1958.
Table 1. North American Spray Dried Blood and Plasma Protein Producers
Table 2. Thermal and solvent resistance of select swine viruses
Source
APCRecommend
Comment
Share
Insta-Pro International
17 de marzo de 2014
Thank you for your comments, too. Interesting study. I've read that transportation (trailers, trucks, etc.) is also being examined. This poses a difficult situation, however - has anyone taken samples from trailers or trucks with PEDv pigs and fed those samples to piglets to see if they develop PEDv? It seems like we're in the same situation here - sure, the trailers test positive, but according to our conversation here, those results are likely meaningless.
It really comes back to good management and cleaning, and IMHO, should happen now. We shouldn't be waiting for a vaccine; this is a crutch. Good management practices should ALWAYS be part of the SOP.
Recommend
Reply
Insta-Pro International
17 de marzo de 2014
Hi Michael,
I agree with all of your comments, but you state "the bioassay results demonstrate that the cells seen in these feed ingredients are not infectious." How can this be known if PEDv is difficult to culture? Wouldn't an improved bioassay involve the use of piglets (I realize this would not be cheap or easy, but with all of the uncertainly surrounding PEDv, may be warranted)?
Thank you.
Recommend
Reply
Insta-Pro International
17 de marzo de 2014
I agree that the PCR test determines presence, but not viability, of PEDv. However, NPPC at the recent PISC conference stated that PEDv is difficult to grow in the lab. From my experience, I would guess that this is true (growing viruses is difficult). Doesn't this increase uncertainty in the transmission route? Couldn't you have a viable but nonculturable situation?
Recommend
Reply
17 de marzo de 2014
From what I have read I do believe there may be significant exposure to PEDv environmentally. Let me be clear, though, that this is likely not the same as with these feed ingredients as PED cells seen environmentally have not undergone the heat treatment common to feeds (which is what inactivates the virus). Good sanitation SOPs are critical.
Recommend
Reply
17 de marzo de 2014
Dave, thanks for keeping this thread going. The University of Minnesota and the USDA Swine Health Monitoring Project both did feeding trials where they fed a control group feed which was inoculated with live virus and the test group which used feed which had tested PCR positive. The control group all got PEDv and the test group did not, thus they each concluded that the PCR positive feed samples did not contain infectious virus. These tests were done independently.
Recommend
Reply
17 de marzo de 2014
I don't think the question is one of whether or not one can grow the virus in the lab. The problem seems to be that some have pointed the finger at feeds which have tested PCR positive for the presence of virus, but the bioassay results demonstrate that the cells seen in these feed ingredients are not infectious. Some normally smart people are talking about continued PCR testing as a litmus test for the suitability of feed ingredients with the result being that some feed ingredients (which test PCR positive, but do not contain infectious cells) may be excluded from the feed. Many of these feed ingredients are helpful to piglets in that they help the digestive system mature faster and healthier and so excluding them could be unhelpful. Beware unintended consequences...
Recommend
Reply
27 de febrero de 2014
The University of Minnesota has published a report of a bioassay to evaluate the infectiousness of feed samples that had tested positive for PED virus by PCR alongside a control group inoculated with three different dilutions of PEDV-
positive material obtained from clinical cases submitted to the Minnesota VDL. The report summarizes as follows: "We were able to reproduce Porcine Epidemic Diarrhea (PED) by oral and intragastric administration of PCR-positive fecal material from PED clinical cases. However, PED was not reproduced in pigs inoculated with feed samples that were positive for PEDV genetic material by PCR. The results from this bioassay indicate that feed can contain PEDV genetic material that is detectable by PCR but is not infectious." The full report can be found at http://www.cvm.umn.edu/sdec/prod/groups/cvm/@pub/@cvm/@sdec/documents/content/cvm_content_447842.pdf
Further, the Swine Health Monitoring Project (apparently involving the USDA) fed four PED PCR positive pig feed samples from two different sources to test viability in a bioassay. The report states "None of the samples tested positive for live PED virus. This conclusion is based on lack of visible severe diarrhea (sporadic, mild diarrhea was observed), no significant decrease in Ct values from inocula (via 1 sample t-test) and no evidence of infection on samples collected at necropsy (PCR and IHC negative." The full report can be found at http://www.cvm.umn.edu/sdec/prod/groups/cvm/@pub/@cvm/@sdec/documents/content/cvm_content_472291.pdf
Why isn't this information getting out there?
Recommend
Reply
26 de febrero de 2014
When will CFIA complete and make public test results which determine if pelleted feed contains infectious virus cells or simply dead virus cells (which show up on PCR tests, leading to statements that these feeds test positive for the PED virus without making a distinction between living (infectious) and dead cells?
Recommend
Reply
Iowa Select Farms
25 de febrero de 2014
I agree that the manufacturing method will kill or inactivate the virus. But biosecurity practice on the plant could be the source of contamination.
We see this every day in swine practice. An example is a filtered farm gets infected with PRRS for failure of biosecurity protocols and not not for the failure of filters or in a truck wash with an excellent clean disinfect and dry procedure can spread PED not for the truck but for a lack of driver biosecurity procedures.
Instead of try to blame we need to work together and consciously do biosecurity assessments an follow solutions.
Recommend
Reply
Biovet Canada
24 de febrero de 2014
http://www.inspection.gc.ca/animals/terrestrial-animals/diseases/other-diseases/ped/2014-02-18/eng/1392762739620/1392762820068
CFIA Statement on Porcine Epidemic Diarrhea Virus in Feed
February 18, 2014: The Canadian Food Inspection Agency (CFIA) is conducting science-based testing to determine if feed may be a contributing factor in the current Porcine Epidemic Diarrhea virus (PEDv) situation.
PED poses no risk to human health or food safety.
Proper biosecurity measures remain the first and best line of defense for pork producers to protect against PED.
As a precautionary measure, on February 9, 2014, Grand Valley Fortifiers issued a voluntary recall for certain pelleted swine nursery feed products containing porcine plasma.
Testing has determined that PED virus was present in samples of US-origin plasma obtained at the third-party manufacturer for Grand Valley Fortifiers. This plasma was used as an ingredient in feed pellets produced by the company. Testing with a swine bioassay has determined that the plasma ingredient contains PED virus capable of causing disease in pigs.
Further testing will be done to assess if the feed pellets are capable of causing disease in piglets, and results are expected within days. Testing will continue to confirm a direct link between the feed and the spread of the disease, as the virus is only confirmed in a single ingredient at this time.
The CFIA is working closely with the company to confirm the effectiveness of the recall, and is closely examining company records to see where potentially affected product was distributed.
The CFIA is also reviewing records of other imports of swine plasma and will work with the Council of Chief Veterinary Officers and the pork industry in Canada to proactively manage the possible risk of transmission through feed.
As the investigation continues, additional actions such as recalls may be necessary to minimize the potential that feed could contribute to the transmission of this disease in Canada.
Recommend
Reply

Would you like to discuss another topic? Create a new post to engage with experts in the community.